Characterizing Heterogeneity in Neuroimaging, Cognition, Clinical Symptoms, and Genetics Among Patients With Late-Life Depression
- PMID: 35262657
- PMCID: PMC8908227
- DOI: 10.1001/jamapsychiatry.2022.0020
Characterizing Heterogeneity in Neuroimaging, Cognition, Clinical Symptoms, and Genetics Among Patients With Late-Life Depression
Abstract
Importance: Late-life depression (LLD) is characterized by considerable heterogeneity in clinical manifestation. Unraveling such heterogeneity might aid in elucidating etiological mechanisms and support precision and individualized medicine.
Objective: To cross-sectionally and longitudinally delineate disease-related heterogeneity in LLD associated with neuroanatomy, cognitive functioning, clinical symptoms, and genetic profiles.
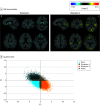
Design, setting, and participants: The Imaging-Based Coordinate System for Aging and Neurodegenerative Diseases (iSTAGING) study is an international multicenter consortium investigating brain aging in pooled and harmonized data from 13 studies with more than 35 000 participants, including a subset of individuals with major depressive disorder. Multimodal data from a multicenter sample (N = 996), including neuroimaging, neurocognitive assessments, and genetics, were analyzed in this study. A semisupervised clustering method (heterogeneity through discriminative analysis) was applied to regional gray matter (GM) brain volumes to derive dimensional representations. Data were collected from July 2017 to July 2020 and analyzed from July 2020 to December 2021.
Main outcomes and measures: Two dimensions were identified to delineate LLD-associated heterogeneity in voxelwise GM maps, white matter (WM) fractional anisotropy, neurocognitive functioning, clinical phenotype, and genetics.
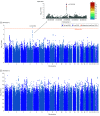
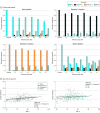
Results: A total of 501 participants with LLD (mean [SD] age, 67.39 [5.56] years; 332 women) and 495 healthy control individuals (mean [SD] age, 66.53 [5.16] years; 333 women) were included. Patients in dimension 1 demonstrated relatively preserved brain anatomy without WM disruptions relative to healthy control individuals. In contrast, patients in dimension 2 showed widespread brain atrophy and WM integrity disruptions, along with cognitive impairment and higher depression severity. Moreover, 1 de novo independent genetic variant (rs13120336; chromosome: 4, 186387714; minor allele, G) was significantly associated with dimension 1 (odds ratio, 2.35; SE, 0.15; P = 3.14 ×108) but not with dimension 2. The 2 dimensions demonstrated significant single-nucleotide variant-based heritability of 18% to 27% within the general population (N = 12 518 in UK Biobank). In a subset of individuals having longitudinal measurements, those in dimension 2 experienced a more rapid longitudinal change in GM and brain age (Cohen f2 = 0.03; P = .02) and were more likely to progress to Alzheimer disease (Cohen f2 = 0.03; P = .03) compared with those in dimension 1 (N = 1431 participants and 7224 scans from the Alzheimer's Disease Neuroimaging Initiative [ADNI], Baltimore Longitudinal Study of Aging [BLSA], and Biomarkers for Older Controls at Risk for Dementia [BIOCARD] data sets).
Conclusions and relevance: This study characterized heterogeneity in LLD into 2 dimensions with distinct neuroanatomical, cognitive, clinical, and genetic profiles. This dimensional approach provides a potential mechanism for investigating the heterogeneity of LLD and the relevance of the latent dimensions to possible disease mechanisms, clinical outcomes, and responses to interventions.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
- U24 AG021886/AG/NIA NIH HHS/United States
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- U01 AG024904/AG/NIA NIH HHS/United States
- G0802594/MRC_/Medical Research Council/United Kingdom
- U19 AG024904/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- RF1 AG054409/AG/NIA NIH HHS/United States
- S10 OD023495/OD/NIH HHS/United States
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- P30 AG072979/AG/NIA NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- R01 NS017950/NS/NINDS NIH HHS/United States
- R01 AG071514/AG/NIA NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- U01 AG052409/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

