Association Between Statin Use and Daptomycin-related Musculoskeletal Adverse Events: A Mixed Approach Combining a Meta-analysis and a Disproportionality Analysis
- PMID: 35262686
- PMCID: PMC9555841
- DOI: 10.1093/cid/ciac128
Association Between Statin Use and Daptomycin-related Musculoskeletal Adverse Events: A Mixed Approach Combining a Meta-analysis and a Disproportionality Analysis
Abstract
Background: There is a growing concern about the association between the combined use of daptomycin (DAP) and statins and the occurrence of musculoskeletal adverse events (MAEs), but this remains controversial. This study aimed to clarify the association between statin use and DAP-related MAEs.
Methods: We used a mixed approach that combines 2 methodologies. First, we conducted a meta-analysis to examine the effects of statin use on DAP-related MAEs. Second, we conducted a disproportionality analysis using the US Food and Drug Administration Adverse Events Reporting System (FAERS) to further confirm the results of the meta-analysis and to examine the effect of each type of statin on DAP-related MAEs in a large population.
Results: In the meta-analysis, statin use significantly increased the incidence of DAP-related rhabdomyolysis (odds ratio [OR]: 3.83; 95% confidence interval [CI]: 1.43-10.26) but not DAP-related myopathy (OR: 1.72; 95% CI: .95-3.12). In the disproportionality analysis using the FAERS, the use of statin significantly increased the reporting OR (ROR) for DAP-related myopathy (ROR: 5.69; 95% CI: 4.31-7.51) and rhabdomyolysis (ROR: 5.77; 95% CI: 4.33-7.68). Atorvastatin, rosuvastatin, and simvastatin all increased the incidence of DAP-related myopathy and rhabdomyolysis.
Conclusion: The mixed approach combining a meta-analysis and disproportionality analysis showed that statin use was associated with the occurrence of DAP-related rhabdomyolysis. The appropriate use of statins and DAP should be performed with careful consideration of its safety.
Keywords: daptomycin; disproportionality analysis; meta-analysis; musculoskeletal adverse event; statin.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. Y. T. received speaker fees from Daiichi-Sankyo Co., Ltd. The other authors have no conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


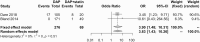
References
-
- Tally FP, DeBruin MF.. Development of daptomycin for gram-positive infections. J Antimicrob Chemother 2000; 46:523–6. - PubMed
-
- Berg ML, Estes LL, Dierkhising RA, Curran B, Enzler MJ.. Evaluation of impact of statin use on development of CPK elevation during daptomycin therapy. Ann Pharmacother 2014; 48:320–7. - PubMed
-
- Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI.. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 2004; 38:1673–81. - PubMed
-
- Parra-Ruiz J, Dueñas-Gutiérrez C, Tomás-Jiménez C, Linares-Palomino JP, Garrido-Gomez J, Hernández-Quero J.. Safety analysis of high dose (>6 mg/kg/day) daptomycin in patients with concomitant statin therapy. Eur J Clin Microbiol Infect Dis 2012; 31:1771–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

