The Role of GJD2(Cx36) in Refractive Error Development
- PMID: 35262731
- PMCID: PMC8934558
- DOI: 10.1167/iovs.63.3.5
The Role of GJD2(Cx36) in Refractive Error Development
Abstract
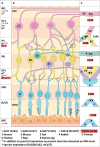
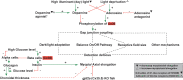
Refractive errors are common eye disorders characterized by a mismatch between the focal power of the eye and its axial length. An increased axial length is a common cause of the refractive error myopia (nearsightedness). The substantial increase in myopia prevalence over the last decades has raised public health concerns because myopia can lead to severe ocular complications later in life. Genomewide association studies (GWAS) have made considerable contributions to the understanding of the genetic architecture of refractive errors. Among the hundreds of genetic variants identified, common variants near the gap junction delta-2 (GJD2) gene have consistently been reported as one of the top hits. GJD2 encodes the connexin 36 (Cx36) protein, which forms gap junction channels and is highly expressed in the neural retina. In this review, we provide current evidence that links GJD2(Cx36) to the development of myopia. We summarize the gap junctional communication in the eye and the specific role of GJD2(Cx36) in retinal processing of visual signals. Finally, we discuss the pathways involving dopamine and gap junction phosphorylation and coupling as potential mechanisms that may explain the role of GJD2(Cx36) in refractive error development.
Conflict of interest statement
Disclosure:
Figures


References
-
- Tideman JWL, Snabel MCC, Tedja MS, et al. .. Association of Axial Length With Risk of Uncorrectable Visual Impairment for Europeans With Myopia. JAMA Ophthalmol. 2016; 134(12): 1355–1363. - PubMed
-
- Verhoeven VJM, Wong KT, Buitendijk GHS, Hofman A, Vingerling JR, Klaver CCW.. Visual consequences of refractive errors in the general population. Ophthalmology. 2015; 122(1): 101–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

