Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC
- PMID: 35262957
- PMCID: PMC9970019
- DOI: 10.1002/hep.32449
Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC
Abstract
Background and aims: Type 3 innate lymphoid cells (ILC3s) are essential for host defense against infection and tissue homeostasis. However, their role in the development of HCC has not been adequately confirmed. In this study, we investigated the immunomodulatory role of short-chain fatty acids (SCFAs) derived from intestinal microbiota in ILC3 regulation.
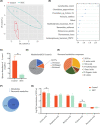
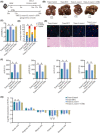
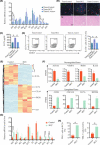
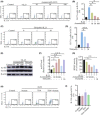
Approach and results: We report that Lactobacillus reuteri was markedly reduced in the gut microbiota of mice with HCC, accompanied by decreased SCFA levels, especially acetate. Additionally, transplantation of fecal bacteria from wild-type mice or L. reuteri could promote an anticancer effect, elevate acetate levels, and reduce IL-17A secretion in mice with HCC. Mechanistically, acetate reduced the production of IL-17A in hepatic ILC3s by inhibiting histone deacetylase activity, increasing the acetylation of SRY (sex-determining region Y)-box transcription factor 13 (Sox13) at site K30, and decreasing expression of Sox13. Moreover, the combination of acetate with programmed death 1/programmed death ligand 1 blockade significantly enhanced antitumor immunity. Consistently, tumor-infiltrating ILC3s correlated with negative prognosis in patients with HCC, which could be functionally mediated by acetate.
Conclusions: These findings suggested that modifying bacteria, changing SCFAs, reducing IL-17A-producing ILC3 infiltration, and combining with immune checkpoint inhibitors will contribute to the clinical treatment of HCC.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The author declares that there is no conflict of interest.
Figures


Comment in
-
Drinking vinegar, a potential adjuvant for immunotherapy of HCC?Hepatology. 2023 Jan 1;77(1):3-5. doi: 10.1002/hep.32463. Epub 2022 Mar 31. Hepatology. 2023. PMID: 35302668 No abstract available.
References
-
- Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–77. - PubMed
-
- Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–32. - PubMed
-
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. . Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

