Biomineralization: Integrating mechanism and evolutionary history
- PMID: 35263127
- PMCID: PMC8906573
- DOI: 10.1126/sciadv.abl9653
Biomineralization: Integrating mechanism and evolutionary history
Abstract
Calcium carbonate (CaCO3) biomineralizing organisms have played major roles in the history of life and the global carbon cycle during the past 541 Ma. Both marine diversification and mass extinctions reflect physiological responses to environmental changes through time. An integrated understanding of carbonate biomineralization is necessary to illuminate this evolutionary record and to understand how modern organisms will respond to 21st century global change. Biomineralization evolved independently but convergently across phyla, suggesting a unity of mechanism that transcends biological differences. In this review, we combine CaCO3 skeleton formation mechanisms with constraints from evolutionary history, omics, and a meta-analysis of isotopic data to develop a plausible model for CaCO3 biomineralization applicable to all phyla. The model provides a framework for understanding the environmental sensitivity of marine calcifiers, past mass extinctions, and resilience in 21st century acidifying oceans. Thus, it frames questions about the past, present, and future of CaCO3 biomineralizing organisms.
Figures
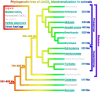


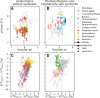
References
-
- Lowenstam H. A., Minerals formed by organisms. Science 211, 1126–1131 (1981). - PubMed
-
- H. A. Lowenstam, S. Weiner, On Biomineralization (Oxford Univ. Press on Demand, 1989).
-
- Olszta M. J., Douglas E. P., Gower L. B., Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer-induced liquid-precursor (PILP) process. Calcif. Tissue Int. 72, 583–591 (2003). - PubMed
-
- Mann S., Molecular recognition in biomineralization. Nature 332, 119 (1988).
-
- Li L., Connors M. J., Kolle M., England G. T., Speiser D. I., Xiao X., Aizenberg J., Ortiz C., Multifunctionality of chiton biomineralized armor with an integrated visual system. Science 350, 952–956 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

