An MKP-MAPK protein phosphorylation cascade controls vascular immunity in plants
- PMID: 35263144
- PMCID: PMC8906744
- DOI: 10.1126/sciadv.abg8723
An MKP-MAPK protein phosphorylation cascade controls vascular immunity in plants
Abstract
Global crop production is greatly reduced by vascular diseases. These diseases include bacterial blight of rice and crucifer black rot caused by Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas campestris pv. campestris (Xcc). The molecular mechanisms that activate vascular defense against such pathogens remains underexplored. Here, we show that an Arabidopsis MAPK phosphatase 1 (MKP1) mutant has increased host susceptibility to the adapted pathogen Xcc and is compromised in nonhost resistance to the rice pathogen Xoo. MKP1 regulates MAPK-mediated phosphorylation of the transcription factor MYB4 that negatively regulates vascular lignification through inhibiting lignin biosynthesis. Induction of lignin biosynthesis is, therefore, an important part of vascular-specific immunity. The role of MKP-MAPK-MYB signaling in lignin biosynthesis and vascular resistance to Xoo is conserved in rice, indicating that these factors form a tissue-specific defense regulatory network. Our study likely reveals a major vascular immune mechanism that underlies tissue-specific disease resistance against bacterial pathogens in plants.
Figures
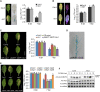
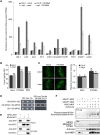
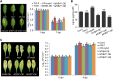

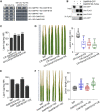
Comment in
-
Change of wind: MKP1 positively regulates vascular immunity.Trends Plant Sci. 2022 Dec;27(12):1193-1195. doi: 10.1016/j.tplants.2022.08.009. Epub 2022 Aug 31. Trends Plant Sci. 2022. PMID: 36057532
Similar articles
-
Rice OsPAD4 functions differently from Arabidopsis AtPAD4 in host-pathogen interactions.Plant J. 2014 May;78(4):619-31. doi: 10.1111/tpj.12500. Epub 2014 Apr 23. Plant J. 2014. PMID: 24617729
-
Loss of chloroplast-localized protein phosphatase 2Cs in Arabidopsis thaliana leads to enhancement of plant immunity and resistance to Xanthomonas campestris pv. campestris infection.Mol Plant Pathol. 2018 May;19(5):1184-1195. doi: 10.1111/mpp.12596. Epub 2017 Nov 2. Mol Plant Pathol. 2018. PMID: 28815858 Free PMC article.
-
Identification and characterization of genes frequently responsive to Xanthomonas oryzae pv. oryzae and Magnaporthe oryzae infections in rice.BMC Genomics. 2020 Jan 6;21(1):21. doi: 10.1186/s12864-019-6438-y. BMC Genomics. 2020. PMID: 31906847 Free PMC article.
-
Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production.BMC Microbiol. 2010 Jul 9;10:187. doi: 10.1186/1471-2180-10-187. BMC Microbiol. 2010. PMID: 20615263 Free PMC article.
-
The OsWRKY6 transcriptional cascade functions in basal defense and Xa1-mediated defense of rice against Xanthomonas oryzae pv. oryzae.Planta. 2022 Jan 25;255(2):47. doi: 10.1007/s00425-022-03830-5. Planta. 2022. PMID: 35076864
Cited by
-
Genome-wide identification and unveiling the role of MAP kinase cascade genes involved in sugarcane response to abiotic stressors.BMC Plant Biol. 2025 Apr 16;25(1):484. doi: 10.1186/s12870-025-06490-1. BMC Plant Biol. 2025. PMID: 40240958 Free PMC article.
-
XYLEM NAC DOMAIN 1 (EjXND1) relieves cold-induced lignification by negatively regulating the EjHB1-EjPRX12 module in loquat fruit.J Adv Res. 2025 Jul;73:93-104. doi: 10.1016/j.jare.2024.08.032. Epub 2024 Sep 2. J Adv Res. 2025. PMID: 39233002 Free PMC article.
-
The FERONIA-RESPONSIVE TO DESICCATION 26 module regulates vascular immunity to Ralstonia solanacearum.Plant Cell. 2024 Dec 23;37(1):koae302. doi: 10.1093/plcell/koae302. Plant Cell. 2024. PMID: 39535787
-
A Cotton Laccase Confers Disease Resistance Against Verticillium dahliae by Promoting Cell Wall Lignification.Mol Plant Pathol. 2025 Jul;26(7):e70125. doi: 10.1111/mpp.70125. Mol Plant Pathol. 2025. PMID: 40657975 Free PMC article.
-
Genome-Wide Investigation and Expression Analysis of the Catalase Gene Family in Oat Plants (Avena sativa L.).Plants (Basel). 2023 Oct 26;12(21):3694. doi: 10.3390/plants12213694. Plants (Basel). 2023. PMID: 37960051 Free PMC article.
References
-
- Lucas W. J., Groover A., Lichtenberger R., Furuta K., Yadav S. R., Helariutta Y., He X. Q., Fukuda H., Kang J., Brady S. M., Patrick J. W., Sperry J., Yoshida A., López-Millán A. F., Grusak M. A., Kachroo P., The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 55, 294–388 (2013). - PubMed
-
- Niño-Liu D. O., Ronald P. C., Bogdanove A. J., Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 7, 303–324 (2006). - PubMed
-
- Guo A., Leach J. E., Examination of rice hydathode water pores exposed to Xanthomonas campestris pv. oryzae. Phytopathology 79, 433–436 (1989).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

