Comparative Study of Bayesian Information Borrowing Methods in Oncology Clinical Trials
- PMID: 35263169
- PMCID: PMC8926037
- DOI: 10.1200/PO.21.00394
Comparative Study of Bayesian Information Borrowing Methods in Oncology Clinical Trials
Abstract
Purpose: With deeper insight into precision medicine, more innovative oncology trial designs have been proposed to contribute to the characteristics of novel antitumor drugs. Bayesian information borrowing is an indispensable part of these designs, which shows great advantages in improving the efficiency of clinical trials. Bayesian methods provide an effective framework when incorporating information. However, the key point lies in how to choose an appropriate method for complex oncology clinical trials.
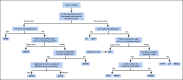
Methods: We divided the borrowing information scenarios into concurrent and nonconcurrent scenarios according to whether the data to be borrowed are observed at the same time as in the current trial or not. Then, we provided an overview of the methods in each scenario. Performance comparison of different methods is carried out with regard to the type I error and power.
Results: As demonstrated by the simulation results in each borrowing scenario, the Bayesian hierarchical model and its extensions are more appropriate for concurrent borrowing. The simulation results demonstrate that the Bayesian hierarchical model shows great advantages when the arms are homogeneous. However, such a method should be adopted with caution when heterogeneity exists. We recommend the other methods, considering heterogeneity. Borrow information from informative priors is more suggested for nonconcurrent borrowing scenarios. Multisource exchangeability models are more suitable for multiple historical trials, while meta-analytic-predictive prior should be carefully applied.
Conclusion: Bayesian information borrowing is useful and can improve the efficiency of clinical trial designs. However, we should carefully choose an appropriate information borrowing method when facing a practical innovative oncology trial, as an appropriate method is essential to provide ideal design performance.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No potential conflicts of interest were reported.
Figures

Similar articles
-
Bayesian semiparametric meta-analytic-predictive prior for historical control borrowing in clinical trials.Stat Med. 2021 Jun 30;40(14):3385-3399. doi: 10.1002/sim.8970. Epub 2021 Apr 13. Stat Med. 2021. PMID: 33851441
-
Bayesian methods for the analysis of early-phase oncology basket trials with information borrowing across cancer types.Stat Med. 2020 Nov 10;39(25):3459-3475. doi: 10.1002/sim.8675. Epub 2020 Jul 27. Stat Med. 2020. PMID: 32717103 Review.
-
Critical appraisal of Bayesian dynamic borrowing from an imperfectly commensurate historical control.Pharm Stat. 2020 Sep;19(5):613-625. doi: 10.1002/pst.2018. Epub 2020 Mar 17. Pharm Stat. 2020. PMID: 32185886
-
Borrowing historical information to improve phase I clinical trials using meta-analytic-predictive priors.J Biopharm Stat. 2022 Jan 2;32(1):34-52. doi: 10.1080/10543406.2022.2058526. Epub 2022 May 20. J Biopharm Stat. 2022. PMID: 35594366
-
Adaptive Bayesian information borrowing methods for finding and optimizing subgroup-specific doses.Clin Trials. 2024 Jun;21(3):308-321. doi: 10.1177/17407745231212193. Epub 2024 Jan 19. Clin Trials. 2024. PMID: 38243401 Free PMC article. Review.
Cited by
-
Optimizing dose-schedule regimens with bayesian adaptive designs: opportunities and challenges.Front Pharmacol. 2023 Nov 23;14:1261312. doi: 10.3389/fphar.2023.1261312. eCollection 2023. Front Pharmacol. 2023. PMID: 38074141 Free PMC article. Review.
-
Bayesian statistics for clinical research.Lancet. 2024 Sep 14;404(10457):1067-1076. doi: 10.1016/S0140-6736(24)01295-9. Lancet. 2024. PMID: 39277290 Review.
-
[Progress and Application of Bayesian Approach in the Early Research and Development of New Anticancer Drugs].Zhongguo Fei Ai Za Zhi. 2022 Oct 20;25(10):730-734. doi: 10.3779/j.issn.1009-3419.2022.102.43. Zhongguo Fei Ai Za Zhi. 2022. PMID: 36285392 Free PMC article. Chinese.
-
New clinical trial design borrowing information across patient subgroups based on fusion-penalized regression models.Stat Methods Med Res. 2024 Oct;33(10):1718-1730. doi: 10.1177/09622802241267355. Epub 2024 Aug 19. Stat Methods Med Res. 2024. PMID: 39158499 Free PMC article.
-
Investigational Use of Real-World Data as a Hybrid Control in Pancreatic Ductal Adenocarcinoma From the Randomized Phase Ib/II MORPHEUS Trial.Clin Pharmacol Ther. 2025 Apr;117(4):1021-1029. doi: 10.1002/cpt.3528. Epub 2024 Dec 20. Clin Pharmacol Ther. 2025. PMID: 39707623 Free PMC article. Clinical Trial.
References
-
- Tang J, Pearce L, O'Donnell-Tormey J, et al. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov. 2018;17:783–784. - PubMed
-
- Bristow RG, Alexander B, Baumann M, et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: A guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018;19:e240–e251. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

