Inhomogeneous Diastereomeric Composition of Mongersen Antisense Phosphorothioate Oligonucleotide Preparations and Related Pharmacological Activity Impairment
- PMID: 35263186
- PMCID: PMC9416542
- DOI: 10.1089/nat.2021.0089
Inhomogeneous Diastereomeric Composition of Mongersen Antisense Phosphorothioate Oligonucleotide Preparations and Related Pharmacological Activity Impairment
Abstract
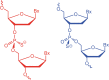
Mongersen is a 21-mer antisense oligonucleotide designed to downregulate Mothers against decapentaplegic homolog 7 (SMAD7) expression to treat Crohn's disease. Mongersen was manufactured in numerous batches at different scales during several years of clinical development, which all appeared identical, using common physicochemical analytical techniques, while only phosphorous-31 nuclear magnetic resonance (31P-NMR) in solution showed marked differences. Close-up analysis of 27 mongersen batches revealed marked differences in SMAD7 downregulation in a cell-based assay. Principal component analysis of 31P-NMR profiles showed strong correlation with SMAD7 downregulation and, therefore, with pharmacological efficacy in vitro. Mongersen contains 20 phosphorothioate (PS) linkages, whose chirality (Rp/Sp) was not controlled during manufacturing. A different diastereomeric composition throughout batches would lead to superimposable analytical data, but to distinct 31P-NMR profiles, as indeed we found. We tentatively suggest that this may be the origin of different biological activity. As similar manifolds are expected for other PS-based oligonucleotides, the protocol described here provides a general method to identify PS chirality issues and a chemometric tool to score each preparation for this elusive feature.
Keywords: 31P-NMR; Crohn's disease; antisense oligonucleotides; mongersen; phosphorothioate.
Conflict of interest statement
M.Mc.N. is an employee of Nogra Pharma Ltd. F.V., S.D., and S.B. are employees of PPM Services SA, Swiss affiliate of Nogra Pharma Ltd. I.C. and A.F. are employees of Regulatory Pharma Net srl (regulatory consultants for the study). G.M., C.S., and L.D.B. have received fees for consultation from PPM Services.
Figures




References
-
- Crooke ST, Baker BF, Crooke RM and Liang XH. (2021). Antisense technology: an overview and prospectus. Nat Rev Drug Discov 20:427–453. - PubMed
-
- Crooke ST, Witztum JL, Bennett CF and Baker BF. (2018) RNA-targeted therapeutics. Cell Metabol 27:714–739. - PubMed
-
- Wan WB and Seth PP. (2016). The medicinal chemistry of therapeutic oligonucleotides. J Med Chem 59:9645–9667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

