Evaluation of Extended Infusion Set Performance in Adults with Type 1 Diabetes: Infusion Set Survival Rate and Glycemic Outcomes from a Pivotal Trial
- PMID: 35263188
- PMCID: PMC9353978
- DOI: 10.1089/dia.2021.0540
Evaluation of Extended Infusion Set Performance in Adults with Type 1 Diabetes: Infusion Set Survival Rate and Glycemic Outcomes from a Pivotal Trial
Abstract
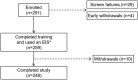
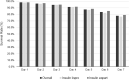
Background: Standard insulin infusion sets (IISs) are to be replaced every 2 to 3 days to avoid complications and diabetic ketosis due to set failure. This pivotal trial evaluated the safety and performance of a new extended-wear infusion set (EIS) when used for 7 days by adults with type 1 diabetes (T1D). Methods: This single-arm, nonrandomized trial enrolled adults (18-80 years of age) with T1D, who used their own MiniMed™ 670G system with insulin lispro or insulin aspart and the EIS for up to 7 days, across 12 consecutive wears. Safety endpoints included incidence of serious adverse events (SAEs), serious adverse device effects (SADEs), unanticipated adverse device effects (UADEs), severe hypoglycemia (SevHypo), severe hyperglycemia (SevHyper), diabetic ketoacidosis (DKA), and skin infection. The EIS failure rate due to unexplained hyperglycemia (i.e., suspected occlusion), the overall EIS survival rate, glycemic control outcomes (i.e., A1C, mean sensor glucose and time spent in established glucose ranges), total daily insulin delivered, and satisfaction with the EIS were determined. Results: The intention to treat population (n = 259, 48% men, 45.0 ± 14.1 years) wore a total of 3041 EIS devices. No SADE, UADE, or DKA events was reported. Overall rates of SAEs, SevHypo, SevHyper, and skin infection were 3.8, 2.5, 104.1, and 20.1 events per 100 participant-years. The rate of EIS failure due to unexplained hyperglycemia at the end of day 7 was 0.1% (95% confidence interval [CI]: 0.03-0.51) and 0.4% (95% CI: 0.16-1.00) for insulin lispro and aspart use, respectively. Overall EIS survival rate at the end of day 7 was 77.8% (95% CI: 76.2-79.3), glycemic control did not change, and participants reported greater satisfaction with the EIS compared with standard IISs worn before the study (P < 0.001). Conclusions: This investigation demonstrates that the EIS, when worn for up to 7 days, was safe and rated with high satisfaction, without adversely affecting glycemic control in adults with T1D. Clinical Trial Registration number: NCT04113694 (https://clinicaltrials.gov/ct2/show/NCT04113694).
Keywords: Adults; Failure rate; Insulin infusion set; Survival rate; Time in range; Unexplained hyperglycemia.
Conflict of interest statement
The investigator authors received research support from Medtronic to conduct the study. S.C., G.Z., J.S., V.C., T.L.C., S.W.L., A.S.R., and R.A.V. are Medtronic employees.
Figures


References
-
- Berg AK, Norgaard K, Thyssen JP, et al. : Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: a cross-sectional study. Diabetes Technol Ther 2018;20:475–482. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

