Induction of broadly neutralizing antibodies using a secreted form of the hepatitis C virus E1E2 heterodimer as a vaccine candidate
- PMID: 35263223
- PMCID: PMC8931252
- DOI: 10.1073/pnas.2112008119
Induction of broadly neutralizing antibodies using a secreted form of the hepatitis C virus E1E2 heterodimer as a vaccine candidate
Abstract
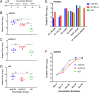
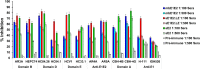
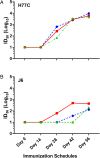
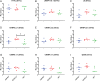
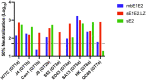
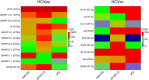
SignificanceHepatitis C virus chronically infects approximately 1% of the world's population, making an effective vaccine for hepatitis C virus a major unmet public health need. The membrane-associated E1E2 envelope glycoprotein has been used in clinical studies as a vaccine candidate. However, limited neutralization breadth and difficulty in producing large amounts of homogeneous membrane-associated E1E2 have hampered efforts to develop an E1E2-based vaccine. Our previous work described the design and biochemical validation of a native-like soluble secreted form of E1E2 (sE1E2). Here, we describe the immunogenic characterization of the sE1E2 complex. sE1E2 elicited broadly neutralizing antibodies in immunized mice, with increased neutralization breadth relative to the membrane-associated E1E2, thereby validating this platform as a promising model system for vaccine development.
Keywords: E1E2 envelope glycoproteins; hepatitis C virus; secreted; vaccine.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2015149118. doi: 10.1073/pnas.2015149118. Proc Natl Acad Sci U S A. 2021. PMID: 33431677 Free PMC article.
-
Fine mapping of murine antibody responses to immunization with a novel soluble form of hepatitis C virus envelope glycoprotein complex.J Virol. 2014 Sep;88(18):10459-71. doi: 10.1128/JVI.01584-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965471 Free PMC article.
-
Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG.J Virol. 2016 Dec 16;91(1):e01552-16. doi: 10.1128/JVI.01552-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795422 Free PMC article.
-
Structural and Biophysical Characterization of the HCV E1E2 Heterodimer for Vaccine Development.Viruses. 2021 May 29;13(6):1027. doi: 10.3390/v13061027. Viruses. 2021. PMID: 34072451 Free PMC article. Review.
-
Hepatitis C Virus E1E2 Structure, Diversity, and Implications for Vaccine Development.Viruses. 2024 May 18;16(5):803. doi: 10.3390/v16050803. Viruses. 2024. PMID: 38793684 Free PMC article. Review.
Cited by
-
Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies.Viruses. 2023 May 11;15(5):1151. doi: 10.3390/v15051151. Viruses. 2023. PMID: 37243237 Free PMC article. Review.
-
Three-Dimensional Reconstruction of the Hepatitis C Virus Envelope Glycoprotein E1E2 Heterodimer by Electron Microscopic Analysis.J Virol. 2023 Jan 31;97(1):e0178822. doi: 10.1128/jvi.01788-22. Epub 2022 Dec 15. J Virol. 2023. PMID: 36519897 Free PMC article.
-
Structure of engineered hepatitis C virus E1E2 ectodomain in complex with neutralizing antibodies.Nat Commun. 2023 Jul 5;14(1):3980. doi: 10.1038/s41467-023-39659-z. Nat Commun. 2023. PMID: 37407593 Free PMC article.
-
Prokaryote- and Eukaryote-Based Expression Systems: Advances in Post-Pandemic Viral Antigen Production for Vaccines.Int J Mol Sci. 2024 Nov 7;25(22):11979. doi: 10.3390/ijms252211979. Int J Mol Sci. 2024. PMID: 39596049 Free PMC article. Review.
-
Glycoengineering of the hepatitis C virus E2 glycoprotein improves biochemical properties and enhances immunogenicity.NPJ Vaccines. 2025 Jun 11;10(1):121. doi: 10.1038/s41541-025-01161-6. NPJ Vaccines. 2025. PMID: 40500295 Free PMC article.
References
-
- WHO, Global Hepatitis Report 2017 (World Health Organization, Geneva, 2017).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

