Microglia regulate chandelier cell axo-axonic synaptogenesis
- PMID: 35263225
- PMCID: PMC8931231
- DOI: 10.1073/pnas.2114476119
Microglia regulate chandelier cell axo-axonic synaptogenesis
Abstract
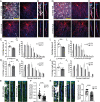
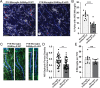
SignificanceChandelier cells (ChCs) are a unique type of GABAergic interneuron that form axo-axonic synapses exclusively on the axon initial segment (AIS) of neocortical pyramidal neurons (PyNs), allowing them to exert powerful yet precise control over PyN firing and population output. The importance of proper ChC function is further underscored by the association of ChC connectivity defects with various neurological conditions. Despite this, the cellular mechanisms governing ChC axo-axonic synapse formation remain poorly understood. Here, we identify microglia as key regulators of ChC axonal morphogenesis and AIS synaptogenesis, and show that disease-induced aberrant microglial activation perturbs proper ChC synaptic development/connectivity in the neocortex. In doing so, such findings highlight the therapeutic potential of manipulating microglia to ensure proper brain wiring.
Keywords: axon initial segment; chandelier cells; inflammation; microglia; synaptogenesis.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Akins M. R., Biederer T., Cell-cell interactions in synaptogenesis. Curr. Opin. Neurobiol. 16, 83–89 (2006). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

