Hypertension induces compensatory left ventricular hypertrophy by a mechanism involving gap junction lateralization and overexpression of CD36, PKC and MMP-2
- PMID: 35263399
- PMCID: PMC9019672
- DOI: 10.47162/RJME.62.3.08
Hypertension induces compensatory left ventricular hypertrophy by a mechanism involving gap junction lateralization and overexpression of CD36, PKC and MMP-2
Abstract
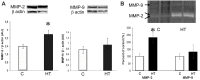
Hypertension-induced left ventricular hypertrophy evolves initially as an adaptive response meant to minimize ventricular wall stress. The mechanisms involved in the preservation of the cardiac function during the "compensatory" phase of the left ventricular hypertrophy are still unclear. Therefore, we aimed at uncovering fine changes that aid the heart to cope with the increased stress in hypertension. Male golden Syrian hamsters were given NG-nitro-L-arginine methyl ester (L-NAME) for 16 weeks, and they became hypertensive (HT), developing left ventricular hypertrophy with no impaired contractility or fibrosis. As compared to age-matched control hamsters, the hypertrophied left ventricles in L-NAME-induced HT hamsters exhibited the following structural and molecular changes: (i) accumulation of lipid droplets (LDs) within cardiomyocytes and relocation of gap junctions to the lateral membrane of cardiomyocytes or close to mitochondria (revealed by electron microscopy); (ii) overexpression of the cluster of differentiation 36 (CD36) fatty acid transporter, protein kinase C (PKC), and matrix metalloproteinase-2 (MMP-2), enhanced activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway, and unchanged expression of the connexin 43 (Cx43) and N-cadherin junctional proteins (assessed by Western blot); (iii) increased protein carbonyl content, assessed with a 2,4-Dinitrophenylhydrazine (DNPH)-based spectrophotometric assay, indicative of an enhanced reactive oxygen species (ROS) production; and (iv) augmented MMP-2 activity (determined by gelatin zymography). These changes may participate in an orchestrated adaptive hypertrophic growth response that helps to maintain cardiac performance, in HT hamsters. Together, these findings could provide support for designing future strategies meant to prevent the transition from compensatory left ventricular hypertrophy to decompensated heart failure.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Figures




References
-
- Habets DDJ, Coumans WA, Voshol PJ, den Boer MAM, Febbraio M, Bonen A, Glatz JFC, Luiken JJFP. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun. 2007;355(1):204–210. - PubMed
-
- Heier C, Haemmerle G. Fat in the heart: the enzymatic machinery regulating cardiac triacylglycerol metabolism. Biochim Biophys Acta. 2016;1861(10):1500–1512. - PubMed
-
- Simon JN, Duglan D, Casadei B, Carnicer R. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J Mol Cell Cardiol. 2014;73:80–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

