Shorter Treatment for Nonsevere Tuberculosis in African and Indian Children
- PMID: 35263517
- PMCID: PMC7612496
- DOI: 10.1056/NEJMoa2104535
Shorter Treatment for Nonsevere Tuberculosis in African and Indian Children
Abstract
Background: Two thirds of children with tuberculosis have nonsevere disease, which may be treatable with a shorter regimen than the current 6-month regimen.
Methods: We conducted an open-label, treatment-shortening, noninferiority trial involving children with nonsevere, symptomatic, presumably drug-susceptible, smear-negative tuberculosis in Uganda, Zambia, South Africa, and India. Children younger than 16 years of age were randomly assigned to 4 months (16 weeks) or 6 months (24 weeks) of standard first-line antituberculosis treatment with pediatric fixed-dose combinations as recommended by the World Health Organization. The primary efficacy outcome was unfavorable status (composite of treatment failure [extension, change, or restart of treatment or tuberculosis recurrence], loss to follow-up during treatment, or death) by 72 weeks, with the exclusion of participants who did not complete 4 months of treatment (modified intention-to-treat population). A noninferiority margin of 6 percentage points was used. The primary safety outcome was an adverse event of grade 3 or higher during treatment and up to 30 days after treatment.
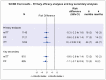
Results: From July 2016 through July 2018, a total of 1204 children underwent randomization (602 in each group). The median age of the participants was 3.5 years (range, 2 months to 15 years), 52% were male, 11% had human immunodeficiency virus infection, and 14% had bacteriologically confirmed tuberculosis. Retention by 72 weeks was 95%, and adherence to the assigned treatment was 94%. A total of 16 participants (3%) in the 4-month group had a primary-outcome event, as compared with 18 (3%) in the 6-month group (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5). The noninferiority of 4 months of treatment was consistent across the intention-to-treat, per-protocol, and key secondary analyses, including when the analysis was restricted to the 958 participants (80%) independently adjudicated to have tuberculosis at baseline. A total of 95 participants (8%) had an adverse event of grade 3 or higher, including 15 adverse drug reactions (11 hepatic events, all but 2 of which occurred within the first 8 weeks, when the treatments were the same in the two groups).
Conclusions: Four months of antituberculosis treatment was noninferior to 6 months of treatment in children with drug-susceptible, nonsevere, smear-negative tuberculosis. (Funded by the U.K. Medical Research Council and others; SHINE ISRCTN number, ISRCTN63579542.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Childhood Tuberculosis - Time for Shorter and Differentiated Treatment.N Engl J Med. 2022 Mar 10;386(10):988-989. doi: 10.1056/NEJMe2200966. N Engl J Med. 2022. PMID: 35263524 No abstract available.
-
Shorter Treatment for Tuberculosis in Children.N Engl J Med. 2022 Jun 23;386(25):2438-2439. doi: 10.1056/NEJMc2204561. N Engl J Med. 2022. PMID: 35731665 No abstract available.
-
Shorter Treatment for Tuberculosis in Children.N Engl J Med. 2022 Jun 23;386(25):2439. doi: 10.1056/NEJMc2204561. N Engl J Med. 2022. PMID: 35731666 No abstract available.
References
-
- WHO. Global Tuberculosis Report 2020. World Health Organization; Geneva: 2020. Licence: CC BY-NC-SA 3.0 IGO 2020.
-
- Wiseman CA, Gie RP, Starke JR, Schaaf HS, Donald PR, Cotton MF, et al. A proposed comprehensive classification of tuberculosis disease severity in children. The Pediatric infectious disease journal. 2012;31(4):347–52. - PubMed
-
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2006;10(7):732–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
