Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar
- PMID: 35263534
- PMCID: PMC8929389
- DOI: 10.1056/NEJMoa2200797
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar
Abstract
Background: Waning of vaccine protection against coronavirus disease 2019 (Covid-19) and the emergence of the omicron (or B.1.1.529) variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have led to expedited efforts to scale up booster vaccination. Protection conferred by booster doses of the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines in Qatar, as compared with protection conferred by the two-dose primary series, is unclear.
Methods: We conducted two matched retrospective cohort studies to assess the effectiveness of booster vaccination, as compared with that of a two-dose primary series alone, against symptomatic SARS-CoV-2 infection and Covid-19-related hospitalization and death during a large wave of omicron infections from December 19, 2021, through January 26, 2022. The association of booster status with infection was estimated with the use of Cox proportional-hazards regression models.
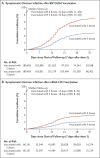
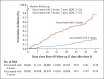
Results: In a population of 2,239,193 persons who had received at least two doses of BNT162b2 or mRNA-1273 vaccine, those who had also received a booster were matched with persons who had not received a booster. Among the BNT162b2-vaccinated persons, the cumulative incidence of symptomatic omicron infection was 2.4% (95% confidence interval [CI], 2.3 to 2.5) in the booster cohort and 4.5% (95% CI, 4.3 to 4.6) in the nonbooster cohort after 35 days of follow-up. Booster effectiveness against symptomatic omicron infection, as compared with that of the primary series, was 49.4% (95% CI, 47.1 to 51.6). Booster effectiveness against Covid-19-related hospitalization and death due to omicron infection, as compared with the primary series, was 76.5% (95% CI, 55.9 to 87.5). BNT162b2 booster effectiveness against symptomatic infection with the delta (or B.1.617.2) variant, as compared with the primary series, was 86.1% (95% CI, 67.3 to 94.1). Among the mRNA-1273-vaccinated persons, the cumulative incidence of symptomatic omicron infection was 1.0% (95% CI, 0.9 to 1.2) in the booster cohort and 1.9% (95% CI, 1.8 to 2.1) in the nonbooster cohort after 35 days; booster effectiveness against symptomatic omicron infection, as compared with the primary series, was 47.3% (95% CI, 40.7 to 53.3). Few severe Covid-19 cases were noted in the mRNA-1273-vaccinated cohorts.
Conclusions: The messenger RNA (mRNA) boosters were highly effective against symptomatic delta infection, but they were less effective against symptomatic omicron infection. However, with both variants, mRNA boosters led to strong protection against Covid-19-related hospitalization and death. (Funded by Weill Cornell Medicine-Qatar and others.).
Copyright © 2022 Massachusetts Medical Society.
Figures

References
-
- World Health Organization. Tracking SARS-CoV-2 variants (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/).
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
