Induction of broadly reactive influenza antibodies increases susceptibility to autoimmunity
- PMID: 35263574
- PMCID: PMC9036619
- DOI: 10.1016/j.celrep.2022.110482
Induction of broadly reactive influenza antibodies increases susceptibility to autoimmunity
Abstract
Infection and vaccination repeatedly expose individuals to antigens that are conserved between influenza virus subtypes. Nevertheless, antibodies recognizing variable influenza epitopes greatly outnumber antibodies reactive against conserved epitopes. Elucidating factors contributing to the paucity of broadly reactive influenza antibodies remains a major obstacle for developing a universal influenza vaccine. Here, we report that inducing broadly reactive influenza antibodies increases autoreactive antibodies in humans and mice and exacerbates disease in four distinct models of autoimmune disease. Importantly, transferring broadly reactive influenza antibodies augments disease in the presence of inflammation or autoimmune susceptibility. Further, broadly reactive influenza antibodies spontaneously arise in mice with defects in B cell tolerance. Together, these data suggest that self-tolerance mechanisms limit the prevalence of broadly reactive influenza antibodies, which can exacerbate disease in the context of additional risk factors.
Keywords: antibody; autoimmunity; influenza; universal influenza vaccine.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.G.T. has consulted for Illumina, 10X, Immunoscape, and Cyotagents. P.G.T. and J.C.C. filed patents related to the treatment of severe respiratory infections (not based on research in this paper).
Figures



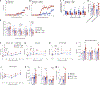
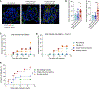

References
-
- Ahmed SS, Schur PH, MacDonald NE, and Steinman L (2014). Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J. Autoimmun. 50, 1–11. - PubMed
-
- Anderson MS, and Bluestone JA (2005). The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

