Fluvoxamine for the Early Treatment of COVID-19: A Meta-analysis of Randomized Clinical Trials
- PMID: 35263710
- PMCID: PMC9128689
- DOI: 10.4269/ajtmh.21-1310
Fluvoxamine for the Early Treatment of COVID-19: A Meta-analysis of Randomized Clinical Trials
Abstract
Fluvoxamine is widely prescribed as an antidepressant. Recent studies show the drug may have a clinical benefit in treating COVID-19. We aimed to perform a meta-analysis of the existing randomized trials of fluvoxamine compared with placebo on the early treatment of COVID-19 patients. We included only randomized clinical trials enrolling ambulatory patients with early-stage disease (symptoms > 7 days) for the prevention of hospitalization. We searched MEDLINE, and clinicaltrials.gov databases to identify trials and extract data with clarifications from the study investigators. We performed a fixed-effects meta-analysis and sensitivity analyses via R to evaluate the pooled estimate of hospitalization. We included three randomized trials: STOP COVID 1 and 2, and the TOGETHER Trial. The studies included a total of 2,196 patients. The STOP COVID trials measured clinical deterioration whereas the TOGETHER Trial measured hospitalization as the primary outcome. All trials reported on hospitalization up to day 28. The meta-analysis results show that patients receiving fluvoxamine were 31% less likely to experience clinical deterioration or hospitalization compared with placebo (risk ratio, 0.69; 95% CI, 0.54-0.88). A sensitivity analysis using the definition of hospitalization resulted in a risk reduction of 21% (95% CI, 0.60-1.03). Data from three randomized controlled trials show that fluvoxamine was associated with a reduction in the primary outcome measure (either clinical deterioration or composite outcome of hospitalization or extended emergency setting observation), although analysis of hospitalization-only was not statistically significant. More evidence from future trials is still needed to support the findings of this meta-analysis.
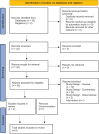
Figures


Similar articles
-
Trial Sequential Analysis and Updated Meta-Analysis of Fluvoxamine on Clinical Deterioration in Adult Patients with Symptomatic COVID-19 Infection.Int J Environ Res Public Health. 2023 Feb 24;20(5):4088. doi: 10.3390/ijerph20054088. Int J Environ Res Public Health. 2023. PMID: 36901099 Free PMC article. Review.
-
The efficacy and safety of fluvoxamine in patients with COVID-19: A systematic review and meta-analysis from randomized controlled trials.PLoS One. 2024 May 16;19(5):e0300512. doi: 10.1371/journal.pone.0300512. eCollection 2024. PLoS One. 2024. PMID: 38753761 Free PMC article.
-
Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis.JAMA Netw Open. 2022 Apr 1;5(4):e226269. doi: 10.1001/jamanetworkopen.2022.6269. JAMA Netw Open. 2022. PMID: 35385087 Free PMC article.
-
Fluvoxamine in Nonhospitalized Patients With Acute COVID-19 Infection and the Lack of Efficacy in Reducing Rates of Hospitalization, Mechanical Ventilation, and Mortality in Placebo-Controlled Trials: A Systematic Review and Meta-Analysis.Am J Ther. 2022 May-Jun 01;29(3):e298-e304. doi: 10.1097/MJT.0000000000001496. Epub 2022 Mar 31. Am J Ther. 2022. PMID: 35383578
-
A systematic review and meta-analysis, investigating dose and time of fluvoxamine treatment efficacy for COVID-19 clinical deterioration, death, and Long-COVID complications.Sci Rep. 2024 Jun 12;14(1):13462. doi: 10.1038/s41598-024-64260-9. Sci Rep. 2024. PMID: 38862591 Free PMC article.
Cited by
-
Trial Sequential Analysis and Updated Meta-Analysis of Fluvoxamine on Clinical Deterioration in Adult Patients with Symptomatic COVID-19 Infection.Int J Environ Res Public Health. 2023 Feb 24;20(5):4088. doi: 10.3390/ijerph20054088. Int J Environ Res Public Health. 2023. PMID: 36901099 Free PMC article. Review.
-
Overview of the potential use of fluvoxamine for COVID-19 and long COVID.Discov Ment Health. 2023;3(1):9. doi: 10.1007/s44192-023-00036-3. Epub 2023 Mar 21. Discov Ment Health. 2023. PMID: 36968793 Free PMC article. Review.
-
Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis.Clin Microbiol Infect. 2023 May;29(5):578-586. doi: 10.1016/j.cmi.2023.01.010. Epub 2023 Jan 16. Clin Microbiol Infect. 2023. PMID: 36657488 Free PMC article.
-
Antidepressant Drugs and COVID-19: A Review of Basic and Clinical Evidence.J Clin Med. 2022 Jul 12;11(14):4038. doi: 10.3390/jcm11144038. J Clin Med. 2022. PMID: 35887802 Free PMC article. Review.
-
Translational Success and Pharmacoeconomic Lessons of Pandemic-Driven Drug Repurposing.Cureus. 2025 May 29;17(5):e85033. doi: 10.7759/cureus.85033. eCollection 2025 May. Cureus. 2025. PMID: 40585709 Free PMC article. Review.
References
-
- Hayashi T Su TP , 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131: 596–610. - PubMed
LinkOut - more resources
Full Text Sources

