Switching to Weekly Lonapegsomatropin from Daily Somatropin in Children with Growth Hormone Deficiency: The fliGHt Trial
- PMID: 35263755
- PMCID: PMC9501775
- DOI: 10.1159/000524003
Switching to Weekly Lonapegsomatropin from Daily Somatropin in Children with Growth Hormone Deficiency: The fliGHt Trial
Abstract
Introduction: The phase 3 fliGHt Trial evaluated the safety and tolerability of once-weekly lonapegsomatropin, a long-acting prodrug, in children with growth hormone deficiency (GHD) who switched from daily somatropin therapy to lonapegsomatropin.
Methods: This multicenter, open-label, 26-week phase 3 trial took place at 28 sites across 4 countries (Australia, Canada, New Zealand, and the USA). The trial enrolled 146 children with GHD, 143 of which were previously treated with daily somatropin. All subjects received once-weekly lonapegsomatropin 0.24 mg human growth hormone/kg/week. The primary outcome measure was safety and tolerability of lonapegsomatropin over 26 weeks. Secondary outcome measures assessed annualized height velocity (AHV), height standard deviation score (SDS), and IGF-1 SDS at 26 weeks.
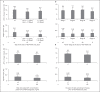
Results: Subjects had a mean prior daily somatropin dose of 0.29 mg/kg/week. Treatment-emergent adverse events (AEs) reported were similar to the published AE profile of daily somatropin therapies. After switching to lonapegsomatropin, the least-squares mean (LSM) AHV was 8.7 cm/year (95% CI: 8.2, 9.2) at Week 26 and LSM height SDS changed from baseline to Week 26 of +0.25 (95% CI: 0.21, 0.29). Among switch subjects, the LSM for average IGF-1 SDS was sustained at Weeks 13 and 26, representing an approximate 0.7 increase from baseline (prior to switching from daily somatropin therapy). Patient-reported outcomes indicated a preference for weekly lonapegsomatropin among both children and their parents.
Conclusions: Lonapegsomatropin treatment outcomes were as expected across a range of ages and treatment experiences. Switching to lonapegsomatropin resulted in a similar AE profile to daily somatropin therapy.
Keywords: Growth hormone; Growth hormone deficiency; Growth hormone replacement therapy; Lonapegsomatropin; Long-acting growth hormone; TransCon human growth hormone.
The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
A.K.M. has received research funding and is an Advisory Board Consultant for Ascendis Pharma, Novo Nordisk, OPKO, and Pfizer and is a speaker for Ascendis Pharma and Novo Nordisk. P.S. has been a speaker for Ascendis Pharma and Novo Nordisk and has received research funding from Ascendis Pharma, OPKO, Novo Nordisk, and Aeterna Zentaris, and is a member of the editorial board of Hormone Research in Paediatrics. K.L.R. has received research funding from Ascendis Pharma, is a speaker for Ascendis, and serves as a board member of The Human Growth Foundation. J.A. has received research funding from Ascendis Pharma, Novo Nordisk, Lumos, Soleno, Rhythm, and Saniona, and has served as a consultant for Consynance Therapeutics and Rhythm. K.A.W. has served as a consultant for Ascendis Pharma. S.J.C. has received research funding from Ascendis Pharma. W.S., M.M., S.D.C., A.S.K., and A.D.S. are employees of Ascendis Pharma, Inc. P.S.T. has received research funding from Ascendis Pharma, Novo Nordisk, Pfizer, and OPKO. U.N., L.D., and L.A.F. have nothing to declare.
Figures
References
-
- Guyda HJ. Four decades of growth hormone therapy for short children: what have we achieved? J Clin Endocrinol Metab. 1999 Dec;84((12)):4307–16. - PubMed
-
- Lustig RH. Optimizing growth hormone efficacy: an evidence-based analysis. Horm Res. 2004;62(Suppl 3):93–7. - PubMed
-
- Carel JC, Ecosse E, Nicolino M, Tauber M, Leger J, Cabrol S, et al. Adult height after long term treatment with recombinant growth hormone for idiopathic isolated growth hormone deficiency: observational follow up study of the French population based registry. BMJ. 2002 Jul 13;325((7355)):70. - PMC - PubMed
-
- Darendeliler F, Lindberg A, Wilton P. Response to growth hormone treatment in isolated growth hormone deficiency versus multiple pituitary hormone deficiency. Horm Res Paediatr. 2011;76(Suppl 1):42–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous