Early changes in laboratory tests predict liver function damage in patients with moderate coronavirus disease 2019: a retrospective multicenter study
- PMID: 35264110
- PMCID: PMC8905025
- DOI: 10.1186/s12876-022-02188-y
Early changes in laboratory tests predict liver function damage in patients with moderate coronavirus disease 2019: a retrospective multicenter study
Abstract
Background: Most patients with coronavirus disease 2019 demonstrate liver function damage. In this study, the laboratory test data of patients with moderate coronavirus disease 2019 were used to establish and evaluate an early prediction model to assess the risk of liver function damage.
Methods: Clinical data and the first laboratory examination results of 101 patients with moderate coronavirus disease 2019 were collected from four hospitals' electronic medical record systems in Jilin Province, China. Data were randomly divided into training and validation sets. A logistic regression analysis was used to determine the independent factors related to liver function damage in patients in the training set to establish a prediction model. Model discrimination, calibration, and clinical usefulness were evaluated in the training and validation sets.
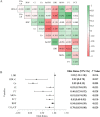
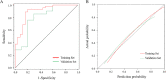
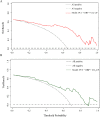
Results: The logistic regression analysis showed that plateletcrit, retinol-binding protein, and carbon dioxide combining power could predict liver function damage (P < 0.05 for all). The receiver operating characteristic curve showed high model discrimination (training set area under the curve: 0.899, validation set area under the curve: 0.800; P < 0.05). The calibration curve showed a good fit (training set: P = 0.59, validation set: P = 0.19; P > 0.05). A decision curve analysis confirmed the clinical usefulness of this model.
Conclusions: In this study, the combined model assesses liver function damage in patients with moderate coronavirus disease 2019 performed well. Thus, it may be helpful as a reference for clinical differentiation of liver function damage. Trial registration retrospectively registered.
Keywords: Carbon dioxide combining power; Coronavirus disease 2019; Liver function; Nomogram; Plateletcrit; Predict; Retinol-binding protein.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Accessed 10 Jan 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

