Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: a Canadian population-based real-world analysis
- PMID: 35264135
- PMCID: PMC8908553
- DOI: 10.1186/s12885-022-09342-5
Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: a Canadian population-based real-world analysis
Abstract
Background: As part of the multi-country I-O Optimise research initiative, this population-based study evaluated real-world treatment patterns and overall survival (OS) in patients treated for advanced non-small cell lung cancer (NSCLC) before and after public reimbursement of immuno-oncology (I-O) therapies in Alberta province, Canada.
Methods: This study used data from the Oncology Outcomes (O2) database, which holds information for ~ 4.5 million residents of Alberta. Eligible patients were adults newly diagnosed with NSCLC between January 2010 and December 2017 and receiving first-line therapy for advanced NSCLC (stage IIIB or IV) either in January 2010-March 2016 (pre-I-O period) or April 2016-June 2019 (post-I-O period). Time periods were based on the first public reimbursement of I-O therapy in Alberta (April 2017), with a built-in 1-year lag time before this date to allow progression to second-line therapy, for which the I-O therapy was indicated. Kaplan-Meier methods were used to estimate OS.
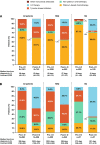
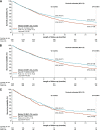
Results: Of 2244 analyzed patients, 1501 (66.9%) and 743 (33.1%) received first-line treatment in the pre-I-O and post-I-O periods, respectively. Between the pre-I-O and post-I-O periods, proportions of patients receiving chemotherapy decreased, with parallel increases in proportions receiving I-O therapies in both the first-line (from < 0.5% to 17%) and second-line (from 8% to 47%) settings. Increased use of I-O therapies in the post-I-O period was observed in subgroups with non-squamous (first line, 15%; second line, 39%) and squamous (first line, 25%; second line, 65%) histology. First-line use of tyrosine kinase inhibitors also increased among patients with non-squamous histology (from 26% to 30%). In parallel with these evolving treatment patterns, median OS increased from 10.2 to 12.1 months for all patients (P < 0.001), from 11.8 to 13.7 months for patients with non-squamous histology (P = 0.022) and from 7.8 to 9.4 months for patients with squamous histology (P = 0.215).
Conclusions: Following public reimbursement, there was a rapid and profound adoption of I-O therapies for advanced NSCLC in Alberta, Canada. In addition, OS outcomes were significantly improved for patients treated in the post-I-O versus pre-I-O periods. These data lend support to the emerging body of evidence for the potential real-world benefits of I-O therapies for treatment of patients with advanced NSCLC.
Keywords: Immune checkpoint inhibitors; Immunotherapy; Non-small cell lung cancer; Population based; Real-world evidence; Retrospective cohort study; Survival; Treatment patterns.
© 2022. The Author(s).
Conflict of interest statement
RC, MJD, JRP, and KL are employees of Bristol Myers Squibb. RC and JRP also report stock ownership in Bristol Myers Squibb. MB, AC, and RM are employees of IQVIA. LL is an employee of Epi-Fit and was contracted (paid) as a consultant by Bristol Myers Squibb to support the I-O Optimise initiative. SK reports no relevant conflicts of interest. WYC reports receipt of research funding from Bristol Myers Squibb.
Figures

References
-
- Canadian Cancer Society. Lung cancer statistics. https://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/.... Accessed 7 Mar 2022.
-
- Herbst RS, Bunn PA., Jr Targeting the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res. 2003;9:5813–5824. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

