Infliximab therapy in refractory sarcoidosis: a multicenter real-world analysis
- PMID: 35264154
- PMCID: PMC8905837
- DOI: 10.1186/s12931-022-01971-5
Infliximab therapy in refractory sarcoidosis: a multicenter real-world analysis
Abstract
Background: Infliximab is a monoclonal antibody that binds and neutralizes circulating tumor necrosis factor-alpha, a key inflammatory cytokine in the pathophysiology of sarcoidosis. Despite the paucity of randomized clinical trials, infliximab is often considered a therapeutic option for refractory disease. Our study aimed to investigate the effectiveness of infliximab in patients with refractory sarcoidosis.
Methods: Sarcoidosis patients from three tertiary centres were retrospectively identified by pharmacy records based on treatment with infliximab. Treatment with Infliximab was initiated in patients who failed first and second line immunomodulators as determined by a multidisciplinary team of Respirologists, Dermatologists, ENT specialists, Rheumatologists, and Neurologists. Participants were characterized by the primary organ for which infliximab was initiated and the total number of organs involved. Clinical outcomes were categorized as treatment success versus failure. We defined treatment success as (A) improvement of cutaneous, upper airway, lymph node, gastrointestinal, eye, or joint manifestations; or (B) improvement or no change in central nervous system (CNS) or pulmonary manifestations.
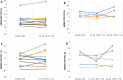
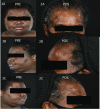
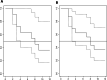
Results: 33 patients with refractory sarcoidosis were identified. The proportion of treatment success was 100% (95% CI 54.1-100) in CNS, 91.7% (95% CI 61.5-99.8) in cutaneous, 78.6% (95% CI 49.2-95.3) in pulmonary and 71.5% (95% CI 29.0-96.3) in upper airway disease. The use of infliximab was associated with a reduction prednisone dose by 50%.
Conclusion: Infliximab is possibly an effective therapy for refractory sarcoidosis, with the greatest value in neurologic and cutaneous manifestations. Across all disease presentations, infliximab facilitated a clinically relevant reduction in corticosteroid dose. Relapse is common after discontinuation of infliximab.
Keywords: Anti-TNF-α; Infliximab; Sarcoidosis.
© 2022. The Author(s).
Conflict of interest statement
NH reports honoraria and grants from Boehringer-Ingelheim, Roche, Bayer, and Janssen, and he also sets on their advisory board. His involvement in such is not related to the manuscript. EA R reports education research grants and lecture fees from Boehringer Ingelheim, Roche and Chiesi outside the field of this review. ML reports honoraria from Abbvie, Amgen and UCB. NK reports grants from BMS (drug only), Sanofi and Abbvie for clinical trials and sets on Roche’s advisory board. MK reports funding for clinical registry from Boehringer Ingelheim and grants from Boehringer Ingelheim, Pieris and Roche. Consulting fees from Boehringer Ingelheim, Roche, Horizon, Cipla, Abbvie, Bellerophon, Algernon and CSL Behring. Lectures\speaker fees from Novartis, Boehringer Ingelheim and Roche. Expert testimony fee from Roche. He sets on data safety monitoring\advisory board of Covance and United Therapeutics. He also reports receiving chief editor allowance from the European Journal of Respirology. The remaining authors declare no conflict of interest.
Figures
References
-
- Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

