Ceria-Zirconia nanoparticles reduce intracellular globotriaosylceramide accumulation and attenuate kidney injury by enhancing the autophagy flux in cellular and animal models of Fabry disease
- PMID: 35264192
- PMCID: PMC8905732
- DOI: 10.1186/s12951-022-01318-8
Ceria-Zirconia nanoparticles reduce intracellular globotriaosylceramide accumulation and attenuate kidney injury by enhancing the autophagy flux in cellular and animal models of Fabry disease
Abstract
Background: Fabry disease (FD) is a lysosome storage disease (LSD) characterized by significantly reduced intracellular autophagy function. This contributes to the progression of intracellular pathologic signaling and can lead to organ injury. Phospholipid-polyethyleneglycol-capped Ceria-Zirconia antioxidant nanoparticles (PEG-CZNPs) have been reported to enhance autophagy flux. We analyzed whether they suppress globotriaosylceramide (Gb3) accumulation by enhancing autophagy flux and thereby attenuate kidney injury in both cellular and animal models of FD.
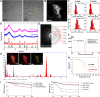
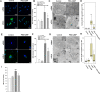
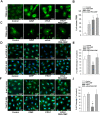
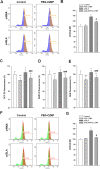
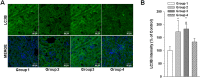
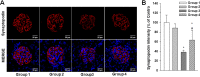
Results: Gb3 was significantly increased in cultured human renal proximal tubular epithelial cells (HK-2) and human podocytes following the siRNA silencing of α galactosidase A (α-GLA). PEG-CZNPs effectively reduced the intracellular accumulation of Gb3 in both cell models of FD and improved both intracellular inflammation and apoptosis in the HK-2 cell model of FD. Moreover these particles attenuated pro fibrotic cytokines in the human podocyte model of FD. This effect was revealed through an improvement of the intracellular autophagy flux function and a reduction in reactive oxygen species (ROS). An FD animal model was generated in which 4-week-old male B6;129-Glatm1Kul/J mice were treated for 8 weeks with 10 mg/kg of PEG-CZNPs (twice weekly via intraperitoneal injection). Gb3 levels were reduced in the kidney tissues of these animals, and their podocyte characteristics and autophagy flux functions were preserved.
Conclusions: PEG-CZNPs alleviate FD associated kidney injury by enhancing autophagy function and thus provide a foundation for the development of new drugs to treat of storage disease.
Keywords: Autophagy flux; Ceria-Zirconia nanoparticle; Fabry disease; Globotriaosylceramide.
© 2022. The Author(s).
Conflict of interest statement
The authors do not have any possible conflict of interests.
Figures






Similar articles
-
PEG-Coated Ceria-Zirconia Nanoparticle Prevent Weight Gain and Obesity-Related Organ Damage Through Autophagy Flux Activation and ROS Scavenging.Int J Nanomedicine. 2025 Jun 26;20:8305-8326. doi: 10.2147/IJN.S510541. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40599398 Free PMC article.
-
Enzyme Replacement Therapy Clears Gb3 Deposits from a Podocyte Cell Culture Model of Fabry Disease but Fails to Restore Altered Cellular Signaling.Cell Physiol Biochem. 2019;52(5):1139-1150. doi: 10.33594/000000077. Cell Physiol Biochem. 2019. PMID: 30990584
-
Ceria-Zirconia Antioxidant Nanoparticles Attenuate Hypoxia-Induced Acute Kidney Injury by Restoring Autophagy Flux and Alleviating Mitochondrial Damage.J Biomed Nanotechnol. 2020 Jul 1;16(7):1144-1159. doi: 10.1166/jbn.2020.2948. J Biomed Nanotechnol. 2020. PMID: 33308381
-
Fabry Nephropathy: An Evidence-Based Narrative Review.Kidney Blood Press Res. 2018;43(2):406-421. doi: 10.1159/000488121. Epub 2018 Mar 16. Kidney Blood Press Res. 2018. PMID: 29558749 Review.
-
Anderson-Fabry disease: a multiorgan disease.Curr Pharm Des. 2013;19(33):5974-96. doi: 10.2174/13816128113199990352. Curr Pharm Des. 2013. PMID: 23448451 Review.
Cited by
-
The role of tubular cells in the pathogenesis of Fabry nephropathy.Front Cardiovasc Med. 2024 Apr 5;11:1386042. doi: 10.3389/fcvm.2024.1386042. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38646152 Free PMC article. Review.
-
PEG-Coated Ceria-Zirconia Nanoparticle Prevent Weight Gain and Obesity-Related Organ Damage Through Autophagy Flux Activation and ROS Scavenging.Int J Nanomedicine. 2025 Jun 26;20:8305-8326. doi: 10.2147/IJN.S510541. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40599398 Free PMC article.
-
The impact of nanomaterials on autophagy across health and disease conditions.Cell Mol Life Sci. 2024 Apr 17;81(1):184. doi: 10.1007/s00018-024-05199-y. Cell Mol Life Sci. 2024. PMID: 38630152 Free PMC article. Review.
-
Salvianolic acid B attenuates membranous nephropathy by activating renal autophagy via microRNA-145-5p/phosphatidylinositol 3-kinase/AKT pathway.Bioengineered. 2022 May;13(5):13956-13969. doi: 10.1080/21655979.2022.2083822. Bioengineered. 2022. PMID: 35723058 Free PMC article.
-
Biochemical Mechanisms beyond Glycosphingolipid Accumulation in Fabry Disease: Might They Provide Additional Therapeutic Treatments?J Clin Med. 2023 Mar 6;12(5):2063. doi: 10.3390/jcm12052063. J Clin Med. 2023. PMID: 36902850 Free PMC article. Review.
References
-
- Ortiz A, Germain DP, Desnick RJ, Politei J, Mauer M, Burlina A, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123(4):416–427. - PubMed
-
- Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med. 2009;11(11):790–796. - PubMed
-
- Schiffmann R, Kopp JB, Austin HA, 3rd, Sabnis S, Moore DF, Weibel T, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285(21):2743–2749. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

