The People's Trial: supporting the public's understanding of randomised trials
- PMID: 35264220
- PMCID: PMC8905031
- DOI: 10.1186/s13063-021-05984-1
The People's Trial: supporting the public's understanding of randomised trials
Abstract
Background: Randomised trials are considered the gold standard in providing robust evidence on the effectiveness of interventions. However, there are relatively few initiatives to help increase public understanding of what randomised trials are and why they are important. This limits the overall acceptance of and public participation in clinical trials. The People's Trial aims to help the public learn about randomised trials, to understand why they matter, and to be better equipped to think critically about health claims by actively involving them in all aspects of trial design. This was done by involving the public in the design, conduct, and dissemination of a randomised trial.
Methods: Using a reflexive approach, we describe the processes of development, conduct, and dissemination of The People's Trial.
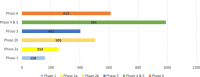
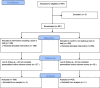
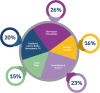
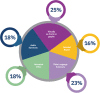
Results: Over 3000 members of the public, from 72 countries, participated in The People's Trial. Through a series of online surveys, the public designed a trial called The Reading Trial. They chose the question the trial would try to answer and decided the components of the trial question. In December 2019, 991 participants were recruited to a trial to answer the question identified and prioritised by the public, i.e. 'Does reading a book in bed make a difference to sleep in comparison with not reading a book in bed?' We report the processes of The People's Trial in seven phases, paralleling the steps of a randomised trial, i.e. question identification and prioritisation, recruitment, randomisation, trial conduct, data analysis, and sharing of findings. We describe the decisions we made, the processes we used, the challenges we encountered, and the lessons we learned.
Conclusion: The People's Trial involved the public successfully in the design, conduct, and dissemination of a randomised trial demonstrating the potential for such initiatives to help the public learn about randomised trials, to understand why they matter, and to be better equipped to think critically about health claims.
Trial registration: ClinicalTrials.gov NCT04185818 . Registered on 4 December 2019.
Keywords: Methodology; Online; Public engagement; Randomised trial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- May M. (2019) Clinical trial costs go under the microscope. Nat Med. 2019; (06 March 2019). https://www.nature.com/articles/d41591-019-00008-7. Accessed 20 July 2021.
-
- Glasziou P, Chalmers I. Research waste is still a scandal—an essay by Paul Glasziou and Iain Chalmers. BMJ. 2018;363:k4645. doi: 10.1136/bmj.k4645. - DOI
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical