Benefits and harms of direct oral anticoagulation and low molecular weight heparin for thromboprophylaxis in patients undergoing non-cardiac surgery: systematic review and network meta-analysis of randomised trials
- PMID: 35264372
- PMCID: PMC8905353
- DOI: 10.1136/bmj-2021-066785
Benefits and harms of direct oral anticoagulation and low molecular weight heparin for thromboprophylaxis in patients undergoing non-cardiac surgery: systematic review and network meta-analysis of randomised trials
Abstract
Objective: To systematically compare the effect of direct oral anticoagulants and low molecular weight heparin for thromboprophylaxis on the benefits and harms to patients undergoing non-cardiac surgery.
Design: Systematic review and network meta-analysis of randomised controlled trials.
Data sources: Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), up to August 2021.
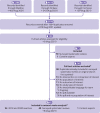
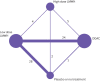
Review methods: Randomised controlled trials in adults undergoing non-cardiac surgery were selected, comparing low molecular weight heparin (prophylactic (low) or higher dose) with direct oral anticoagulants or with no active treatment. Main outcomes were symptomatic venous thromboembolism, symptomatic pulmonary embolism, and major bleeding. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for network meta-analyses. Abstracts and full texts were screened independently in duplicate. Data were abstracted on study participants, interventions, and outcomes, and risk of bias was assessed independently in duplicate. Frequentist network meta-analysis with multivariate random effects models provided odds ratios with 95% confidence intervals, and GRADE (grading of recommendations, assessment, development, and evaluation) assessments indicated the certainty of the evidence.
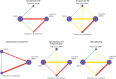
Results: 68 randomised controlled trials were included (51 orthopaedic, 10 general, four gynaecological, two thoracic, and one urological surgery), involving 45 445 patients. Low dose (odds ratio 0.33, 95% confidence interval 0.16 to 0.67) and high dose (0.19, 0.07 to 0.54) low molecular weight heparin, and direct oral anticoagulants (0.17, 0.07 to 0.41) reduced symptomatic venous thromboembolism compared with no active treatment, with absolute risk differences of 1-100 per 1000 patients, depending on baseline risks (certainty of evidence, moderate to high). None of the active agents reduced symptomatic pulmonary embolism (certainty of evidence, low to moderate). Direct oral anticoagulants and low molecular weight heparin were associated with a 2-3-fold increase in the odds of major bleeding compared with no active treatment (certainty of evidence, moderate to high), with absolute risk differences as high as 50 per 1000 in patients at high risk. Compared with low dose low molecular weight heparin, high dose low molecular weight heparin did not reduce symptomatic venous thromboembolism (0.57, 0.26 to 1.27) but increased major bleeding (1.87, 1.06 to 3.31); direct oral anticoagulants reduced symptomatic venous thromboembolism (0.53, 0.32 to 0.89) and did not increase major bleeding (1.23, 0.89 to 1.69).
Conclusions: Direct oral anticoagulants and low molecular weight heparin reduced venous thromboembolism compared with no active treatment but probably increased major bleeding to a similar extent. Direct oral anticoagulants probably prevent symptomatic venous thromboembolism to a greater extent than prophylactic low molecular weight heparin.
Systematic review registration: PROSPERO CRD42018106181.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no specific support for the submitted work; PDV received support from the Canadian Urological Association Scholarship Foundation for his work relevant to this study; FG’s institution received research funding from F Hoffmann-La Roche, Novo Nordisk, Takeda, Pfizer, and Bayer, all outside the submitted work; PJD reports grants from Abbott Diagnostics, Boehringer-Ingelheim, Roche Diagnostics, and Siemens and non-financial support from Philips Healthcare outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
