Achieving pain control in early rheumatoid arthritis with baricitinib monotherapy or in combination with methotrexate versus methotrexate monotherapy
- PMID: 35264432
- PMCID: PMC8915362
- DOI: 10.1136/rmdopen-2021-001994
Achieving pain control in early rheumatoid arthritis with baricitinib monotherapy or in combination with methotrexate versus methotrexate monotherapy
Abstract
Objectives: This post hoc analysis assessed speed, magnitude and maintenance of pain improvement in patients with early rheumatoid arthritis (RA) receiving baricitinib, baricitinib and methotrexate (MTX), or MTX over 1 year. Cumulative pain and quality of life benefits were also assessed.
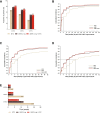
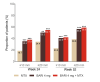
Methods: Randomised, double-blind, phase 3 study RA-BEGIN (NCT01711359) compared baricitinib 4 mg (N=159), baricitinib 4 mg +MTX (N=215) and MTX (N=210) in patients with RA who had no or limited prior disease-modifying antirheumatic drug treatment. Pain was assessed on a 0-100 mm Visual Analogue Scale (VAS). Proportion of patients with ≥30%, ≥50% and ≥70% pain improvement from baseline; ≤20 mm and ≤10 mm on the pain VAS; and time to achieve pain improvement thresholds were assessed over 52 weeks, as were Patient Global Assessment (PtGA) and 36-Item Short Form Health Survey Physical Component Score (SF-36 PCS) outcomes.
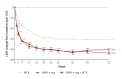
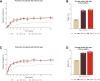
Results: Baricitinib monotherapy or combination with MTX provides greater (least square mean changes (LSM) from baseline -40 mm and -43 mm, respectively) and more rapid (median 12 and 8 weeks to ≥70% improvement, respectively) pain relief than MTX alone (LSM -31 mm, median 20 weeks to ≥70% improvement) over 52 weeks. Baricitinib, alone or combination, provides 9-10 additional weeks of limited to no pain, similar gain in achievable wellness measured through PtGA, and 5-7 additional weeks with change in SF-36 PCS ≥5 vs MTX over 1 year.
Conclusions: Patients treated with baricitinib reported significantly greater and more rapid pain relief, more weeks with limited to no pain, and clinically meaningful improvements in physical health than patients treated with MTX alone over 1 year.
Keywords: arthritis, rheumatoid; patient reported outcome measures; therapeutics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PCT has received research grants and/or consultation fees from Galapagos, Gilead, Eli Lilly and Company, AbbVie, and Pfizer. Rieke Alten has received honoraria and research grants from Abbvie, BMS, Galalapagos, Gilead, Janssen, Lilly, MSD, Novartis and Pfizer. JMAG has received consulting fees from Eli Lilly and Company and Pfizer; honoraria from Abbvie, Eli Lilly and Company, Galapagos, Gilead, Pfizer, Roche and UCB; and support for attending meetings from Abbvie, MSD, Pfizer, Roche and UCB. YK has received grants or speaking fees from AbbVie, Astellas, Ayumi, Bristol–Myers Squibb, Chugai, Eisai, Eli Lilly and Company, Jansen, Kissei, Kirin, Novartis pharma, Pfizer, Sanofi, Takeda, Tanabe-Mitsubishi and UCB. CW, AQ, BJ, NB and JRT are employees and may be shareholders of Eli Lilly and Company. RF has received grants from AbbVie, Eli Lilly and Company, Gilead, and Pfizer; consulting fees from AbbVie, Eli Lilly and Company, Gilead, and Pfizer; and participated on a Data Safety Monitoring Board or Advisory Board for AbbVie, Eli Lilly and Company, Gilead and Pfizer.
Figures


References
-
- Kosinski M, Kujawski SC, Martin R, et al. Health-Related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 2002;8:231–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
