SGSM2 inhibits thyroid cancer progression by activating RAP1 and enhancing competitive RAS inhibition
- PMID: 35264562
- PMCID: PMC8907342
- DOI: 10.1038/s41419-022-04598-y
SGSM2 inhibits thyroid cancer progression by activating RAP1 and enhancing competitive RAS inhibition
Abstract
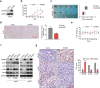
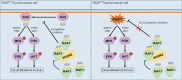
Thyroid cancer (TC) is one of the most common malignancies involving the head and neck, and its incidences are increasing every year. Small G protein signaling modulators 2 (SGSM2) belongs to a newly identified protein group that contributes to numerous cancer progression. However, its role in TC remains unknown. The aim of this study was to explore the functions and underlying molecular mechanism of SGSM2 in the progression of thyroid tumorigenesis. Here, we demonstrated that SGSM2 expression was markedly decreased in TC, and that lower SGSM2 expression was potentially related to worse patient prognosis. Meanwhile, the SGSM2 levels were not directly correlated with BRAF or RAS mutations in TC. Based on our functional analysis, ectopic SGSM2 expression strongly prevented cell proliferation, migration, invasion, and tumorigenic activity in TC cells that harbored wild type RAS. Mechanistically, we demonstrated that SGSM2 interacted with Small G protein Ras-associated protein 1(RAP1) and augmented its activity. Activated RAP1 then competitively suppressed RAS activation and thereby downregulated output of MAPK/ERK and PI3K/Akt networks, which are primary contributors of TC. In summary, the present study reports a tumor suppressive role of SGSM2 in TC. Moreover, we revealed the underlying molecular mechanism, thus providing a potential therapeutic target for TCs that harbor wild type RAS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Targeting RAS-MAPK-ERK and PI3K-AKT-mTOR signal transduction pathways to chemosensitize anaplastic thyroid carcinoma.Transl Res. 2014 Nov;164(5):411-23. doi: 10.1016/j.trsl.2014.06.005. Epub 2014 Jun 23. Transl Res. 2014. PMID: 25016932
-
Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.Thyroid. 2010 Jul;20(7):697-706. doi: 10.1089/thy.2010.1646. Thyroid. 2010. PMID: 20578891 Free PMC article. Review.
-
Small G protein signalling modulator 2 (SGSM2) is involved in oestrogen receptor-positive breast cancer metastasis through enhancement of migratory cell adhesion via interaction with E-cadherin.Cell Adh Migr. 2019 Dec;13(1):120-137. doi: 10.1080/19336918.2019.1568139. Epub 2019 Feb 11. Cell Adh Migr. 2019. PMID: 30744493 Free PMC article.
-
TRIM29 promotes progression of thyroid carcinoma via activating P13K/AKT signaling pathway.Oncol Rep. 2017 Mar;37(3):1555-1564. doi: 10.3892/or.2017.5364. Epub 2017 Jan 13. Oncol Rep. 2017. PMID: 28098872
-
[Intracellular signaling mechanisms in thyroid cancer].Cir Cir. 2016 Sep-Oct;84(5):434-43. doi: 10.1016/j.circir.2016.05.017. Epub 2016 Aug 8. Cir Cir. 2016. PMID: 27423883 Review. Spanish.
Cited by
-
Zyxin Inhibits the Proliferation, Migration, and Invasion of Osteosarcoma via Rap1-Mediated Inhibition of the MEK/ERK Signaling Pathway.Biomedicines. 2023 Aug 21;11(8):2314. doi: 10.3390/biomedicines11082314. Biomedicines. 2023. PMID: 37626810 Free PMC article.
-
Deciphering the role of PLCD3 in lung cancer: A gateway to glycolytic reprogramming via PKC-Rap1 activation.Heliyon. 2024 Aug 30;10(17):e37063. doi: 10.1016/j.heliyon.2024.e37063. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296221 Free PMC article.
-
CD147 regulates the Rap1 signaling pathway to promote proliferation, migration, and invasion, and inhibit apoptosis in colorectal cancer cells.Sci Rep. 2025 Apr 20;15(1):13647. doi: 10.1038/s41598-025-98266-8. Sci Rep. 2025. PMID: 40254691 Free PMC article.
-
Molecular Mechanisms Associated with the Development of the Metritis Complex in Dairy Cattle.Genes (Basel). 2024 Mar 30;15(4):439. doi: 10.3390/genes15040439. Genes (Basel). 2024. PMID: 38674374 Free PMC article.
-
Advances in targeted therapy and biomarker research in thyroid cancer.Front Endocrinol (Lausanne). 2024 Mar 4;15:1372553. doi: 10.3389/fendo.2024.1372553. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38501105 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

