Bone marrow-independent adventitial macrophage progenitor cells contribute to angiogenesis
- PMID: 35264563
- PMCID: PMC8907187
- DOI: 10.1038/s41419-022-04605-2
Bone marrow-independent adventitial macrophage progenitor cells contribute to angiogenesis
Abstract
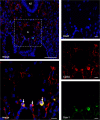
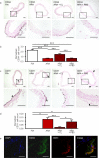
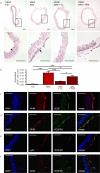
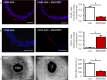
Pathological angiogenesis promotes tumor growth, metastasis, and atherosclerotic plaque rupture. Macrophages are key players in these processes. However, whether these macrophages differentiate from bone marrow-derived monocytes or from local vascular wall-resident stem and progenitor cells (VW-SCs) is an unresolved issue of angiogenesis. To answer this question, we analyzed vascular sprouting and alterations in aortic cell populations in mouse aortic ring assays (ARA). ARA culture leads to the generation of large numbers of macrophages, especially within the aortic adventitia. Using immunohistochemical fate-mapping and genetic in vivo-labeling approaches we show that 60% of these macrophages differentiate from bone marrow-independent Ly6c+/Sca-1+ adventitial progenitor cells. Analysis of the NCX-/- mouse model that genetically lacks embryonic circulation and yolk sac perfusion indicates that at least some of those progenitor cells arise yolk sac-independent. Macrophages represent the main source of VEGF in ARA that vice versa promotes the generation of additional macrophages thereby creating a pro-angiogenetic feedforward loop. Additionally, macrophage-derived VEGF activates CD34+ progenitor cells within the adventitial vasculogenic zone to differentiate into CD31+ endothelial cells. Consequently, depletion of macrophages and VEGFR2 antagonism drastically reduce vascular sprouting activity in ARA. In summary, we show that angiogenic activation induces differentiation of macrophages from bone marrow-derived as well as from bone marrow-independent VW-SCs. The latter ones are at least partially yolk sac-independent, too. Those VW-SC-derived macrophages critically contribute to angiogenesis, making them an attractive target to interfere with pathological angiogenesis in cancer and atherosclerosis as well as with regenerative angiogenesis in ischemic cardiovascular disorders.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. - PubMed
-
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–6. - PubMed
-
- Subhani S, Vavilala DT, Mukherji M. HIF inhibitors for ischemic retinopathies and cancers: options beyond anti-VEGF therapies. Angiogenesis. 2016;19:257–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

