Bacterial ribosome collision sensing by a MutS DNA repair ATPase paralogue
- PMID: 35264791
- PMCID: PMC9041291
- DOI: 10.1038/s41586-022-04487-6
Bacterial ribosome collision sensing by a MutS DNA repair ATPase paralogue
Abstract
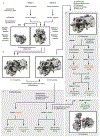
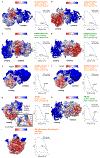
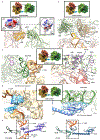
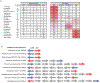
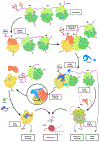
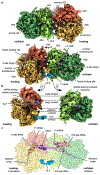
Ribosome stalling during translation is detrimental to cellular fitness, but how this is sensed and elicits recycling of ribosomal subunits and quality control of associated mRNA and incomplete nascent chains is poorly understood1,2. Here we uncover Bacillus subtilis MutS2, a member of the conserved MutS family of ATPases that function in DNA mismatch repair3, as an unexpected ribosome-binding protein with an essential function in translational quality control. Cryo-electron microscopy analysis of affinity-purified native complexes shows that MutS2 functions in sensing collisions between stalled and translating ribosomes and suggests how ribosome collisions can serve as platforms to deploy downstream processes: MutS2 has an RNA endonuclease small MutS-related (SMR) domain, as well as an ATPase/clamp domain that is properly positioned to promote ribosomal subunit dissociation, which is a requirement both for ribosome recycling and for initiation of ribosome-associated protein quality control (RQC). Accordingly, MutS2 promotes nascent chain modification with alanine-tail degrons-an early step in RQC-in an ATPase domain-dependent manner. The relevance of these observations is underscored by evidence of strong co-occurrence of MutS2 and RQC genes across bacterial phyla. Overall, the findings demonstrate a deeply conserved role for ribosome collisions in mounting a complex response to the interruption of translation within open reading frames.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures









Comment in
-
Ribosome collisions: New ways to initiate ribosome rescue.Curr Biol. 2022 May 23;32(10):R469-R472. doi: 10.1016/j.cub.2022.04.038. Curr Biol. 2022. PMID: 35609545 Free PMC article.
References
-
- Joazeiro CAP Ribosomal Stalling During Translation: Providing Substrates for Ribosome-Associated Protein Quality Control. Annu. Rev. Cell Dev. Biol 33, 343–368 (2017). - PubMed
-
- Moore SD & Sauer RT The tmRNA system for translational surveillance and ribosome rescue. Annu. Rev. Biochem 76, 101–24 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

