Somatostatin Receptor 2: A Potential Predictive Biomarker for Immune Checkpoint Inhibitor Treatment
- PMID: 35264912
- PMCID: PMC8898825
- DOI: 10.3389/pore.2022.1610196
Somatostatin Receptor 2: A Potential Predictive Biomarker for Immune Checkpoint Inhibitor Treatment
Abstract
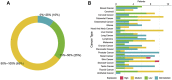
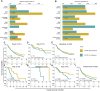
Somatostatin receptor 2 (SSTR2), the most abundant receptor of somatostatin (SST), possesses immunoreactivity and is altered in many cancers. However, the association between SSTR2 and efficacy of immune checkpoint inhibitors (ICIs) has not yet been reported. Immunohistochemistry (IHC) information across 20 cancers was collected from the Human Protein Atlas (HPA) and used to analyze the expression of SSTR2. Immune signatures collected from public databases, such as BioCarta or Reactome, were used to investigate the association between SSTR2 and the tumor microenviroment in the Cancer Genome Atlas (TCGA). Data from cohorts treated with ICIs were collected to assess whether SSTR2 is associated with benefits from ICIs treatment. In the HPA, we found the SSTR2 IHC-positive rate of 13 cancers to be above 50%. Five types of cancer express SSTR2 mildly (positive rate: 25%-50%), while the remaining two types of cancer barely stained SSTR2-positive (positive rate: 0%-24%). In TCGA analysis, immune cell signatures and immune function pathways were enriched in high SSTR2 expression groups in most cancers. In each ICIs treated cohort, patients with high SSTR2 expression experienced numerically superior objective response rate (Braun: 14.8% vs 13.4%, p = 0.85; Gide: 69.4% vs 40.5%, p = 0.025; Mariathasan: 22.4% vs 16.7%, p = 0.233; Miao: 37.5% vs 11.8%; Riaz: 32.0% vs 7.7%, p = 0.067) and overall survival (Braun: HR (95%CI): 0.80 [0.62-1.04], p = 0.80; Gide: HR (95%CI): 0.61 [0.29-1.30], p = 0.20; Mariathasan: HR (95%CI): 0.83 [0.64-1.08], p = 0.16; Miao: HR (95%CI): 0.24 [0.086-0.65], p = 0.0028; Nathanson cohort: HR (95%CI): 0 [0-inf], p = 0.18; Riaz: HR (95%CI): 0.24 [0.086-0.65], p = 0.028) than patients with low SSTR2 expression. In pooled cohort, we found these differences were significant (Pool: 24.6% vs 16.7%, p = 0.0077; HR (95% CI): 0.77 [0.65-0.91], p = 0.0018). Our results suggest that SSTR2 is a potential predictive biomarker for response to ICIs.
Keywords: bioinformatics; immune checkpoint inhibitors; predictive biomarker; somatostatin receptor 2; tumor microenvironment.
Copyright © 2022 Wang, Yuan, Chu, Gao, Jin, Jia and Zhu.
Conflict of interest statement
Author ZJ was employed by the company GloriousMed Clinical Laboratory (Shanghai) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in Patients with Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed Following Treatment with Platinum-Based Chemotherapy: a Single-Arm, Multicentre, Phase 2 Trial. The Lancet (2016) 387:1909–20. 10.1016/S0140-6736(16)00561-4 - DOI - PMC - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-small-cell Lung Cancer (KEYNOTE-042): a Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393:1819–30. 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

