Engineered Biological Neural Networks on High Density CMOS Microelectrode Arrays
- PMID: 35264928
- PMCID: PMC8900719
- DOI: 10.3389/fnins.2022.829884
Engineered Biological Neural Networks on High Density CMOS Microelectrode Arrays
Abstract
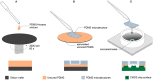
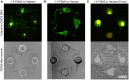
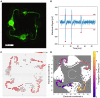
In bottom-up neuroscience, questions on neural information processing are addressed by engineering small but reproducible biological neural networks of defined network topology in vitro. The network topology can be controlled by culturing neurons within polydimethylsiloxane (PDMS) microstructures that are combined with microelectrode arrays (MEAs) for electric access to the network. However, currently used glass MEAs are limited to 256 electrodes and pose a limitation to the spatial resolution as well as the design of more complex microstructures. The use of high density complementary metal-oxide-semiconductor (CMOS) MEAs greatly increases the spatial resolution, enabling sub-cellular readout and stimulation of neurons in defined neural networks. Unfortunately, the non-planar surface of CMOS MEAs complicates the attachment of PDMS microstructures. To overcome the problem of axons escaping the microstructures through the ridges of the CMOS MEA, we stamp-transferred a thin film of hexane-diluted PDMS onto the array such that the PDMS filled the ridges at the contact surface of the microstructures without clogging the axon guidance channels. This method resulted in 23 % of structurally fully connected but sealed networks on the CMOS MEA of which about 45 % showed spiking activity in all channels. Moreover, we provide an impedance-based method to visualize the exact location of the microstructures on the MEA and show that our method can confine axonal growth within the PDMS microstructures. Finally, the high spatial resolution of the CMOS MEA enabled us to show that action potentials follow the unidirectional topology of our circular multi-node microstructure.
Keywords: CMOS; PDMS microstructures; bottom-up neuroscience; engineered networks; in vitro; microelectrode arrays.
Copyright © 2022 Duru, Küchler, Ihle, Forró, Bernardi, Girardin, Hengsteler, Wheeler, Vörös and Ruff.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abbott S. (2018). Printing Science: Principles and Practice. Ipswich.
-
- Aebersold M. J., Dermutz H., Forró C., Weydert S., Thompson-Steckel G., Vörös J., et al. . (2016). “brains on a chip”: towards engineered neural networks. TrAC Trends Anal. Chem. 78, 60–69. 10.1016/j.trac.2016.01.025 - DOI
LinkOut - more resources
Full Text Sources

