Honokiol Ameliorates Post-Myocardial Infarction Heart Failure Through Ucp3-Mediated Reactive Oxygen Species Inhibition
- PMID: 35264952
- PMCID: PMC8899544
- DOI: 10.3389/fphar.2022.811682
Honokiol Ameliorates Post-Myocardial Infarction Heart Failure Through Ucp3-Mediated Reactive Oxygen Species Inhibition
Erratum in
-
Corrigendum: Honokiol ameliorates post-myocardial infarction heart failure through Ucp3-mediated reactive oxygen species inhibition.Front Pharmacol. 2022 Sep 27;13:1000887. doi: 10.3389/fphar.2022.1000887. eCollection 2022. Front Pharmacol. 2022. PMID: 36238543 Free PMC article.
Abstract
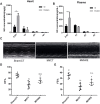
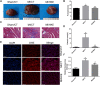
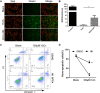
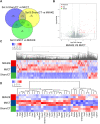
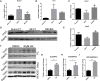
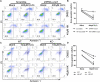
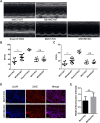
Post-myocardial infarction heart failure (post-MI HF) is one of the leading global causes of death, and current prevention and treatment methods still cannot avoid the increasing incidence. Honokiol (HK) has previously been reported to improve myocardial ischemia/reperfusion injury and reverse myocardial hypertrophy by activating Sirt1 and Sirt3. We suspect that HK may also have a therapeutic effect on post-MI HF. In this study, we aimed to investigate the efficacy and mechanism of HK in the treatment of post-MI HF. We found that HK inhibited myocardial reactive oxygen species (ROS) production, reduced myocardial fibrosis, and improved cardiac function in mice after MI. HK also reduced the abnormality of mitochondrial membrane potential (MMP) and apoptosis of cardiomyocytes caused by peroxide in neonatal cardiomyocytes. RNAseq results revealed that HK restored the transcriptome changes to a certain extent and significantly enhanced the expression of mitochondrial inner membrane uncoupling protein isoform 3 (Ucp3), a protein that inhibits the production of mitochondrial ROS, protects cardiomyocytes, and relieves heart failure after myocardial infarction (MI). In cardiomyocytes with impaired Ucp3 expression, HK cannot protect against the damage caused by peroxide. More importantly, in Ucp3 knockout mice, HK did not change the increase in the ROS level and cardiac function damage after MI. Taken together, our results suggest that HK can increase the expression of the cardioprotective protein Ucp3 and maintain MMP, thereby inhibiting the production of ROS after MI and ameliorating heart failure.
Keywords: UCP3; heart failure; honokiol; myocardial infarction; reactive oxygen species.
Copyright © 2022 Liu, Tang, Li, Su, Zhu, Dou, Liu, Pei, Yang, Ye and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

