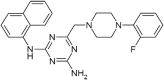
Structure-Activity Relationship Studies of 4-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-6-imino-N-(naphthalen-2-yl)-1,3,5-triazin-2-amine (FPMINT) Analogues as Inhibitors of Human Equilibrative Nucleoside Transporters
- PMID: 35264969
- PMCID: PMC8899516
- DOI: 10.3389/fphar.2022.837555
Structure-Activity Relationship Studies of 4-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-6-imino-N-(naphthalen-2-yl)-1,3,5-triazin-2-amine (FPMINT) Analogues as Inhibitors of Human Equilibrative Nucleoside Transporters
Abstract
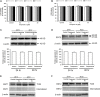
Equilibrative nucleoside transporters (ENTs) play a vital role in nucleotide synthesis, regulation of adenosine function and chemotherapy. Current inhibitors of ENTs are mostly ENT1-selective. Our previous study has demonstrated that 4-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-6-imino-N-(naphthalen-2-yl)-1,3,5-triazin-2-amine (FPMINT) is a novel inhibitor of ENTs, which is more selective to ENT2 than to ENT1. The present study aimed to screen a series of FPMINT analogues and study their structure-activity relationship. Nucleoside transporter-deficient cells transfected with cloned human ENT1 and ENT2 were used as in vitro models. The results of the [3H]uridine uptake study showed that the replacement of the naphthalene moiety with the benzene moiety could abolish the inhibitory effects on ENT1 and ENT2. The addition of chloride to the meta position of this benzene moiety could restore only the inhibitory effect on ENT1 but had no effect on ENT2. However, the addition of the methyl group to the meta position or the ethyl or oxymethyl group to the para position of this benzene moiety could regain the inhibitory activity on both ENT1 and ENT2. The presence of a halogen substitute, regardless of the position, in the fluorophenyl moiety next to the piperazine ring was essential for the inhibitory effects on ENT1 and ENT2. Among the analogues tested, compound 3c was the most potent inhibitor. Compound 3c reduced V max of [3H]uridine uptake in ENT1 and ENT2 without affecting K m. The inhibitory effect of compound 3c could not be washed out. Compound 3c did not affect cell viability, protein expression and internalization of ENT1 and ENT2. Therefore, similar to FPMINT, compound 3c was an irreversible and non-competitive inhibitor. Molecular docking analysis also showed that the binding site of compound 3c in ENT1 may be different from that of other conventional inhibitors. It is expected that structural modification may further improve its potency and selectivity and lead to the development of useful pharmacological agents.
Keywords: FPMINT; equilibrative nucleoside transporter; inhibitor; mechanism of action; structure-activity relationship.
Copyright © 2022 Li, Mak, Li, Zheng, Shiu, Seto, Lee and Leung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Baldwin S. A., Yao S. Y., Hyde R. J., Ng A. M., Foppolo S., Barnes K., et al. (2005). Functional Characterization of Novel Human and Mouse Equilibrative Nucleoside Transporters (hENT3 and mENT3) Located in Intracellular Membranes. J. Biol. Chem. 280 (16), 15880–15887. 10.1074/jbc.M414337200 - DOI - PubMed
-
- Barnes K., Dobrzynski H., Foppolo S., Beal P. R., Ismat F., Scullion E. R., et al. (2006). Distribution and Functional Characterization of Equilibrative Nucleoside Transporter-4, a Novel Cardiac Adenosine Transporter Activated at Acidic pH. Circ. Res. 99 (5), 510–519. 10.1161/01.RES.0000238359.18495.42 - DOI - PubMed
LinkOut - more resources
Full Text Sources

