Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance
- PMID: 35264975
- PMCID: PMC8899333
- DOI: 10.3389/fphys.2021.793251
Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance
Abstract
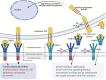
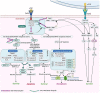
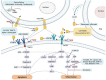
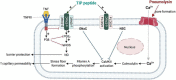
Alveolar-capillary leak is a hallmark of the acute respiratory distress syndrome (ARDS), a potentially lethal complication of severe sepsis, trauma and pneumonia, including COVID-19. Apart from barrier dysfunction, ARDS is characterized by hyper-inflammation and impaired alveolar fluid clearance (AFC), which foster the development of pulmonary permeability edema and hamper gas exchange. Tumor Necrosis Factor (TNF) is an evolutionarily conserved pleiotropic cytokine, involved in host immune defense against pathogens and cancer. TNF exists in both membrane-bound and soluble form and its mainly -but not exclusively- pro-inflammatory and cytolytic actions are mediated by partially overlapping TNFR1 and TNFR2 binding sites situated at the interface between neighboring subunits in the homo-trimer. Whereas TNFR1 signaling can mediate hyper-inflammation and impaired barrier function and AFC in the lungs, ligand stimulation of TNFR2 can protect from ventilation-induced lung injury. Spatially distinct from the TNFR binding sites, TNF harbors within its structure a lectin-like domain that rather protects lung function in ARDS. The lectin-like domain of TNF -mimicked by the 17 residue TIP peptide- represents a physiological mediator of alveolar-capillary barrier protection. and increases AFC in both hydrostatic and permeability pulmonary edema animal models. The TIP peptide directly activates the epithelial sodium channel (ENaC) -a key mediator of fluid and blood pressure control- upon binding to its α subunit, which is also a part of the non-selective cation channel (NSC). Activity of the lectin-like domain of TNF is preserved in complexes between TNF and its soluble TNFRs and can be physiologically relevant in pneumonia. Antibody- and soluble TNFR-based therapeutic strategies show considerable success in diseases such as rheumatoid arthritis, psoriasis and inflammatory bowel disease, but their chronic use can increase susceptibility to infection. Since the lectin-like domain of TNF does not interfere with TNF's anti-bacterial actions, while exerting protective actions in the alveolar-capillary compartments, it is currently evaluated in clinical trials in ARDS and COVID-19. A more comprehensive knowledge of the precise role of the TNFR binding sites versus the lectin-like domain of TNF in lung injury, tissue hypoxia, repair and remodeling may foster the development of novel therapeutics for ARDS.
Keywords: COVID-19; TNF lectin-like domain; TNF receptor; acute respiratory distress syndrome; epithelial sodium channel.
Copyright © 2022 Lucas, Hadizamani, Enkhbaatar, Csanyi, Caldwell, Hundsberger, Sridhar, Lever, Hudel, Ash, Ushio-Fukai, Fukai, Chakraborty, Verin, Eaton, Romero and Hamacher.
Conflict of interest statement
RL is inventor of several patents relating to the use of the TNF-derived TIP peptide in pulmonary edema reabsorption. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abraham E., Anzueto A., Gutierrez G., Tessler S., San Pedro G., Wunderink R., et al. (1998). Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet, 351, 929–933. 10.1016/S0140-6736(05)60602-2 - DOI - PubMed
-
- Abraham E., Glauser M. P., Butler T., Garbino J., Gelmont D., Laterre P. F., et al. (1997). p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA 277, 1531–1538. 10.1001/jama.1997.03540430043031 - DOI - PubMed
-
- Abraham E., Laterre P. F., Garbino J., Pingleton S., Butler T., Dugernier T., et al. (2001). Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit. Care Med. 29 503–510. 10.1097/00003246-200103000-00006 - DOI - PubMed
-
- Abraham E., Wunderink R., Silverman H., Perl T. M., Nasraway S., Levy H., et al. (1995). Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome: a randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb sepsis study group. JAMA 273 934–941. 10.1001/jama.1995.03520360048038 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

