Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1
- PMID: 35265193
- PMCID: PMC8899590
- DOI: 10.7150/thno.68864
Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1
Abstract
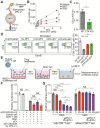
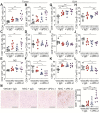
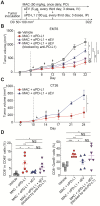
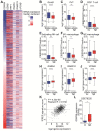
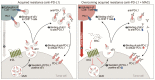
Extracellular vesicles (EVs) carrying tumor cell-derived programmed death-ligand 1 (PD-L1) interact with programmed death 1 (PD-1)-producing T cells, thus significantly lowering a patient's response to immune checkpoint blockade drugs. No drug that reinvigorates CD8+ T cells by suppressing EV PD-L1 has been approved for clinical usage. Here we have identified macitentan (MAC), an FDA-approved oral drug, as a robust booster of antitumor responses in CD8+ T cells by suppressing tumor cell-derived EV PD-L1. Methods: EV was analyzed by the data from nanoparticle tracking, immunoblotting analyses, and nano-flow cytometry. Antitumor immunity was evaluated by luciferase assay and immune phenotyping using flow cytometry. Clinical relevance was analyzed using the cancer genome atlas database. Results: MAC inhibited secretion of tumor-derived EV PD-L1 by targeting the endothelin receptor A (ETA) in breast cancer cells and xenograft models. MAC enhanced CD8+ T cell-mediated tumor killing by decreasing the binding of PD-1 to the EV PD-L1 and thus synergizing the effects of the anti-PD-L1 antibody. MAC also showed an anticancer effect in triple-negative breast cancer (TNBC)-bearing immunocompetent mice but not in nude mice. The combination therapy of MAC and anti-PD-L1 antibody significantly improved antitumor efficacy by increasing CD8+ T cell number and activity with decreasing Treg number in the tumors and draining lymph nodes in TNBC, colon, and lung syngeneic tumor models. The antitumor effect of MAC was reversed by injecting exogenous EV PD-L1. Notably, ETA level was strongly associated with the innate anti-PD-1 resistance gene signature and the low response to the PD-1/PD-L1 blockade. Conclusion: These findings strongly demonstrate that MAC, already approved for clinical applications, can be used to improve and/or overcome the inadequate response to PD-1/PD-L1 blockade therapy.
Keywords: Cancer; Exosome; Extracellular vesicle; PD-L1; immunotherapy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

