Heterogeneity of tyrosine-based melanin anabolism regulates pulmonary and cerebral organotropic colonization microenvironment of melanoma cells
- PMID: 35265199
- PMCID: PMC8899568
- DOI: 10.7150/thno.69198
Heterogeneity of tyrosine-based melanin anabolism regulates pulmonary and cerebral organotropic colonization microenvironment of melanoma cells
Abstract
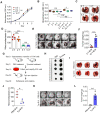
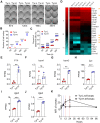
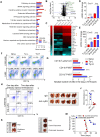
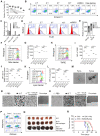
Background: Dietary tyrosine regulating melanoma progression has been well-recognized. However, whether tyrosine-based melanin anabolism contributes to pulmonary and cerebral organotropic colonization of melanoma remains elusive. Furthermore, approaches based on targeting tyrosinase activity to inhibiting multi-organ metastasis of melanoma cells need to be designed and validated. Methods: Patients derived melanoma cells and mouse B16 melanoma cells with different pigmentation were employed in this investigation. Tyrosine content dynamics in tumors and multiple organs during the melanoma progression was monitored, and tyrosine-based melanin synthesis of melanoma cells derived from multi-organ was determined. Additionally, we also adopted RNA-seq, flow cytometry, real-time PCR and composite metastasis mouse model to analyze organotropic colonization and to validate designed therapeutic strategies. Results: B16 melanoma cells with high activity of tyrosinase and sensitivity of tyrosine utilization for melanin synthesis (Tyr-H cells) easily colonized in the lung, while B16 melanoma cells lacking above characteristics (Tyr-L cells) exhibited potent proliferation in the brain. Mechanistically, Tyr-H cells recruited and trained neutrophils and macrophages to establish pulmonary metastatic niche dependent on highly secreted CXCL1 and CXCL2 and an excessive melanosome accumulation-induced cell death. Tyr-L cells enhanced PD-L1 expression in tumor-infiltrated macrophages when they are progressing in the brain. Accordingly, intervention of tyrosinase activity (2-Ethoxybenzamide or hydroquinone) in combination with inhibitors of phagocytosis (GSK343) or chemotaxis (SB225002) suppressed organotropic colonization and significantly improved the survival of melanoma- bearing mice treated with immune checkpoint blockade (PD1 antibody). Conclusions: The heterogeneity of melanoma cells in utilization of tyrosine is associated with organotropic colonization, providing the basis for developing new strategies to combat melanoma.
Keywords: Melanoma; immune checkpoint; melanin anabolism; organotropic colonization; tumor heterogeneity.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Neural stem cells inhibit melanin production by activation of Wnt inhibitors.J Dermatol Sci. 2013 Dec;72(3):274-83. doi: 10.1016/j.jdermsci.2013.08.006. Epub 2013 Aug 23. J Dermatol Sci. 2013. PMID: 24016750
-
Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells.Steroids. 2011 Nov;76(12):1297-304. doi: 10.1016/j.steroids.2011.06.008. Epub 2011 Jun 30. Steroids. 2011. PMID: 21745488
-
Palmitoyl copper peptide and acetyl tyrosine complex enhances melanin production in both A375 and B16 cell lines.Biochem Biophys Res Commun. 2025 Jan;742:151060. doi: 10.1016/j.bbrc.2024.151060. Epub 2024 Nov 28. Biochem Biophys Res Commun. 2025. PMID: 39632290
-
Quantitative proteomic analysis uncovers inhibition of melanin synthesis by silk fibroin via MITF/tyrosinase axis in B16 melanoma cells.Life Sci. 2021 Nov 1;284:119930. doi: 10.1016/j.lfs.2021.119930. Epub 2021 Sep 1. Life Sci. 2021. PMID: 34480938
-
Targeting Melanin in Melanoma with Radionuclide Therapy.Int J Mol Sci. 2022 Aug 23;23(17):9520. doi: 10.3390/ijms23179520. Int J Mol Sci. 2022. PMID: 36076924 Free PMC article. Review.
Cited by
-
Identification of CD8+ T-cell exhaustion signatures for prognosis in HBV-related hepatocellular carcinoma patients by integrated analysis of single-cell and bulk RNA-sequencing.BMC Cancer. 2024 Jan 10;24(1):53. doi: 10.1186/s12885-023-11804-3. BMC Cancer. 2024. PMID: 38200408 Free PMC article.
-
Biosynthesis, Characterization, and Bioactivity of L-Alanyl-L-tyrosine in Promoting Melanin Synthesis.Appl Biochem Biotechnol. 2024 Jul;196(7):3693-3707. doi: 10.1007/s12010-023-04713-5. Epub 2023 Sep 15. Appl Biochem Biotechnol. 2024. PMID: 37713063
-
Causal associations of BAFF-R on IgD+ CD24- B cell immune cell trait with hepatocellular carcinoma and the mediating role of phenylacetylglutamate levels: a Mendelian randomization study.J Cancer. 2024 Jun 24;15(14):4591-4603. doi: 10.7150/jca.96059. eCollection 2024. J Cancer. 2024. PMID: 39006080 Free PMC article.
-
Hepatocellular carcinoma escapes immune surveillance through deceiving thymus into recalling peripheral activated CD8+ T cells.Neoplasia. 2025 Sep;67:101210. doi: 10.1016/j.neo.2025.101210. Epub 2025 Jul 18. Neoplasia. 2025. PMID: 40683125 Free PMC article.
-
Signature of immune-related metabolic genes predicts the prognosis of hepatocellular carcinoma.Front Immunol. 2024 Nov 25;15:1481331. doi: 10.3389/fimmu.2024.1481331. eCollection 2024. Front Immunol. 2024. PMID: 39654885 Free PMC article.
References
-
- Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33:2413–22. - PubMed
-
- Kudchadkar RR, Lowe MC, Khan MK, McBrien SM. Metastatic melanoma. CA Cancer J Clin. 2020;70:78–85. - PubMed
-
- Brodt P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res. 2016;22:5971–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

