Platinum-based drugs for cancer therapy and anti-tumor strategies
- PMID: 35265202
- PMCID: PMC8899578
- DOI: 10.7150/thno.69424
Platinum-based drugs for cancer therapy and anti-tumor strategies
Abstract
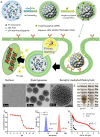
Platinum-based drugs cisplatin, carboplatin, and oxaliplatin are widely used for chemotherapeutic eradication of cancer. However, the side effects of platinum drugs, such as lack of selectivity, high systemic toxicity, and drug resistance, seriously limit their clinical application. With advancements in nanotechnology and chemical synthesis, Pt-based anti-cancer drugs have made great progress in cancer therapy in recent years. Many strategies relied on the anti-cancer mechanism similar to cisplatin and achieved some success by modifying existing platinum drugs. Pt-based nanodrugs, such as platinum nanoclusters, have novel anti-cancer mechanisms and great potential in tumor-targeted therapy and have shown promising results in clinical application. In this review, we systematically explored the development of first-line platinum chemotherapy drugs in the clinic and their anti-cancer mechanisms. We also summarize the progress of Pt-based anti-cancer drug application in cancer therapy, emphasizing their modification to enhance the anti-tumor effect. Finally, we address challenges faced by platinum chemotherapy drugs, especially Pt nanocluster-based nanodrugs, in cancer treatment. The new platinum drugs and their targeted modifications undoubtedly provide a promising prospect for improving the current anti-cancer treatments.
Keywords: Anti-cancer mechanism; Cancer therapy; Platinum nanoclusters; Platinum-based drugs; Systemic toxicity.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

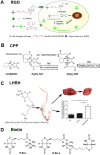

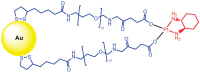
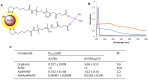



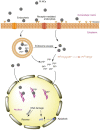


References
-
- Bian M, Fan R, Zhao S, Liu W. Targeting the thioredoxin system as a strategy for cancer therapy. J Med Chem. 2019;62:7309–7321. - PubMed
-
- Zhang J, Li X, Han X, Liu R, Fang J. Targeting the thioredoxin system for cancer therapy. Trends Pharmacol Sci. 2017;38:794–808. - PubMed
-
- Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2020;21:37–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

