Efficacy of Sequential Capecitabine on Adjuvant Chemotherapy of Triple-Negative Breast Cancer
- PMID: 35265304
- PMCID: PMC8901322
- DOI: 10.1155/2022/7430775
Efficacy of Sequential Capecitabine on Adjuvant Chemotherapy of Triple-Negative Breast Cancer
Abstract
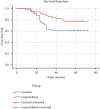
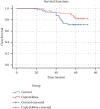
This paper aims to evaluate the efficacy of capecitabine as extended adjuvant treatment after anthracycline and paclitaxel combined adjuvant chemotherapy for women with early triple-negative breast cancer (TNBC). The patients with early TNBC were randomly assigned to capecitabine sequential treatment for 4 cycles and without any sequential treatment in the control group after anthracycline and paclitaxel combined adjuvant chemotherapy. The primary end point was disease-free survival (DFS). The secondary end point was overall survival (OS). One hundred patients were enrolled in this study between June 2013 and February 2015. Median age was 49 years ranging from 25 to 66 years and treatment was well tolerance. The median follow-up time after random allocation was 58 months (range: 11-62 months). There was no significant difference in DFS and OS between the two groups (hazard ratio (HR) of DFS was 0.50; 95% CI, 0.24-1.05; P=0.066). Our study shows that although the addition of four cycles capecitabine after anthracycline and paclitaxel combining adjuvant chemotherapy does not improve DFS and OS, but the trend of DFS is improved. The possible reason is that the four-cycle treatment of capecitabine is not enough, and another possible reason is that the number of cases is not enough.
Copyright © 2022 Xun Xi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Adjuvant addition of capecitabine to early-stage triple-negative breast cancer patients receiving standard chemotherapy: a meta-analysis.Breast Cancer Res Treat. 2020 Feb;179(3):533-542. doi: 10.1007/s10549-019-05513-4. Epub 2019 Dec 21. Breast Cancer Res Treat. 2020. PMID: 31865475 Review.
-
Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01).J Clin Oncol. 2020 Jan 20;38(3):203-213. doi: 10.1200/JCO.19.00904. Epub 2019 Dec 5. J Clin Oncol. 2020. PMID: 31804894 Free PMC article. Clinical Trial.
-
The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Jan 19;21(1):78. doi: 10.1186/s12885-021-07791-y. BMC Cancer. 2021. PMID: 33468087 Free PMC article.
-
Adjuvant Capecitabine With Docetaxel and Cyclophosphamide Plus Epirubicin for Triple-Negative Breast Cancer (CBCSG010): An Open-Label, Randomized, Multicenter, Phase III Trial.J Clin Oncol. 2020 Jun 1;38(16):1774-1784. doi: 10.1200/JCO.19.02474. Epub 2020 Apr 10. J Clin Oncol. 2020. PMID: 32275467 Free PMC article. Clinical Trial.
-
First efficacy results of capecitabine with anthracycline- and taxane-based adjuvant therapy in high-risk early breast cancer: a meta-analysis.PLoS One. 2012;7(3):e32474. doi: 10.1371/journal.pone.0032474. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396769 Free PMC article.
References
-
- Miwa M., Ura M., Nishida M., et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. European Journal of Cancer . 1998;34(8):1274–1281. doi: 10.1016/s0959-8049(98)00058-6. - DOI - PubMed
-
- O’Shaughnessy J., Miles D., Vukelja S., et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. Journal of Clinical Oncology . 2002;20(12):2812–2823. doi: 10.1200/JCO.2002.09.002. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

