Maslinic Acid Protects against Streptozotocin-Induced Diabetic Retinopathy by Activating Nrf2 and Suppressing NF- κ B
- PMID: 35265366
- PMCID: PMC8901311
- DOI: 10.1155/2022/3044202
Maslinic Acid Protects against Streptozotocin-Induced Diabetic Retinopathy by Activating Nrf2 and Suppressing NF- κ B
Abstract
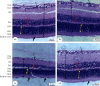
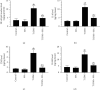
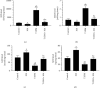
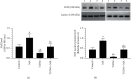
This study tested the protective effect of maslinic acid (MA) against diabetic retinopathy (DR) in rats with type 1 diabetes mellitus (T1DM) and investigated possible mechanisms of action. DM was introduced by streptozotocin (STZ) (65 mg/kg, i.p.). Control and STZ (T1DM) were divided into 2 subgroups, which received either the vehicle or MA (80 mg/kg). Serum, pancreases, and retinas were collected for further use. MA significantly reduced fasting glucose levels in the control and T1DM rats but enhanced fasting insulin levels and partially increased the size of the islets of Langerhans and the number of β-cells in T1DM rats. In addition, MA significantly improved the retina structure by preventing the reduction in the area between the inner and outer limiting membranes (ILM and OLM, respectively) and increasing the number of cells forming the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). Associated with these effects, MA significantly reduced the total levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), as well as the nuclear levels of NF-κB p65, mRNA levels of Bax, and protein levels of cleaved caspase-3 in the retinas of T1DM rats. However, MA significantly lowered levels of reactive oxygen species (ROS) and malondialdehyde (MDA) but significantly increased the nuclear levels of Nrf2, protein levels of Bcl2, and total levels of superoxide dismutase (SOD) and reduced glutathione (GSH) in the retinas of the control and T1DM rats. In conclusion, MA prevents DR by antioxidant potential mediated by the activation of Nrf2.
Copyright © 2022 Nasser A. Alsabaani et al.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures

References
-
- Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Research and Clinical Practice . 2019;157(594) doi: 10.1016/j.diabres.2019.107843.107843 - DOI - PubMed
-
- Stewart M. W. Pathophysiology of diabetic retinopathy. Diabetic Retinopathy . 2010:1–30. doi: 10.1007/978-0-387-85900-2_1. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

