Effective Delivery of siRNA-Loaded Nanoparticles for Overcoming Oxaliplatin Resistance in Colorectal Cancer
- PMID: 35265524
- PMCID: PMC8898837
- DOI: 10.3389/fonc.2022.827891
Effective Delivery of siRNA-Loaded Nanoparticles for Overcoming Oxaliplatin Resistance in Colorectal Cancer
Erratum in
-
Corrigendum: Effective Delivery of siRNA-Loaded Nanoparticles for Overcoming Oxaliplatin Resistance in Colorectal Cancer.Front Oncol. 2022 Jun 27;12:916983. doi: 10.3389/fonc.2022.916983. eCollection 2022. Front Oncol. 2022. PMID: 35837110 Free PMC article.
Abstract
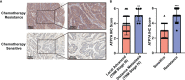
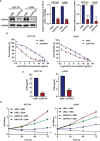
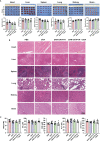
Chemotherapy resistance represents a formidable obstacle in advanced or metastatic colorectal cancer (CRC) patients. It is reported that ATPase copper transporting alpha (ATP7A) plays an important role in chemotherapy resistance in CRC. Here, we identified ATP7A as a potentially key gene of OXA resistance in CRC. The patients with higher expression of ATP7A tended to have platinum drug resistance. While the lower expression of ATP7A by siRNA knockdown resulted in enhancement of OXA sensitivity and increased OXA-induced apoptosis. Further, we demonstrated a novel and safe strategy to increase CRC chemosensitivity by delivering siRNA into tumor cells via a novel nanoparticle, DAN. In summary, our study provided a novel nanocarrier-based delivery of ATP7A to interfere in a key gene of chemo-resistance in CRC, which may be a novel therapeutic strategy to overcome chemotherapy resistance in CRC.
Keywords: ATP7A; chemoresistance; colorectal cancer; oxaliplatin; siRNA delivery.
Copyright © 2022 Zhou, Zhang, Wang, Huang and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Suppression MGP inhibits tumor proliferation and reverses oxaliplatin resistance in colorectal cancer.Biochem Pharmacol. 2021 Jul;189:114390. doi: 10.1016/j.bcp.2020.114390. Epub 2020 Dec 22. Biochem Pharmacol. 2021. PMID: 33359068
-
Precision medicine-guided co-delivery of ASPN siRNA and oxaliplatin by nanoparticles to overcome chemoresistance of colorectal cancer.Biomaterials. 2022 Nov;290:121827. doi: 10.1016/j.biomaterials.2022.121827. Epub 2022 Sep 27. Biomaterials. 2022. PMID: 36228517
-
Corrigendum: Effective Delivery of siRNA-Loaded Nanoparticles for Overcoming Oxaliplatin Resistance in Colorectal Cancer.Front Oncol. 2022 Jun 27;12:916983. doi: 10.3389/fonc.2022.916983. eCollection 2022. Front Oncol. 2022. PMID: 35837110 Free PMC article.
-
Biological landscape and nanostructural view in development and reversal of oxaliplatin resistance in colorectal cancer.Transl Oncol. 2024 Feb;40:101846. doi: 10.1016/j.tranon.2023.101846. Epub 2023 Dec 1. Transl Oncol. 2024. PMID: 38042134 Free PMC article. Review.
-
Copper efflux transporters ATP7A and ATP7B: Novel biomarkers for platinum drug resistance and targets for therapy.IUBMB Life. 2018 Mar;70(3):183-191. doi: 10.1002/iub.1722. Epub 2018 Feb 2. IUBMB Life. 2018. PMID: 29394468 Review.
Cited by
-
Carbon Dots-Based Nanozyme for Drug-Resistant Lung Cancer Therapy by Encapsulated Doxorubicin/siRNA Cocktail.Int J Nanomedicine. 2023 Feb 21;18:933-948. doi: 10.2147/IJN.S390984. eCollection 2023. Int J Nanomedicine. 2023. PMID: 36852185 Free PMC article.
-
Pan-Cancer Analysis Reveals SH3TC2 as an Oncogene for Colorectal Cancer and Promotes Tumorigenesis via the MAPK Pathway.Cancers (Basel). 2022 Jul 31;14(15):3735. doi: 10.3390/cancers14153735. Cancers (Basel). 2022. PMID: 35954399 Free PMC article.
-
Copper in colorectal cancer: From copper-related mechanisms to clinical cancer therapies.Clin Transl Med. 2024 Jun;14(6):e1724. doi: 10.1002/ctm2.1724. Clin Transl Med. 2024. PMID: 38804588 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

