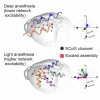
Slow waves form expanding, memory-rich mesostates steered by local excitability in fading anesthesia
- PMID: 35265807
- PMCID: PMC8899414
- DOI: 10.1016/j.isci.2022.103918
Slow waves form expanding, memory-rich mesostates steered by local excitability in fading anesthesia
Abstract
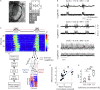
In the arousal process, the brain restores its integrative activity from the synchronized state of slow wave activity (SWA). The mechanisms underpinning this state transition remain, however, to be elucidated. Here we simultaneously probed neuronal assemblies throughout the whole cortex with micro-electrocorticographic recordings in mice. We investigated the progressive shaping of propagating SWA at different levels of isoflurane. We found a form of memory of the wavefront shapes at deep anesthesia, tightly alternating posterior-anterior-posterior patterns. At low isoflurane, metastable patterns propagated in more directions, reflecting an increased complexity. The wandering across these mesostates progressively increased its randomness, as predicted by simulations of a network of spiking neurons, and confirmed in our experimental data. The complexity increase is explained by the elevated excitability of local assemblies with no modifications of the network connectivity. These results shed new light on the functional reorganization of the cortical network as anesthesia fades out.
Keywords: Experimental models in systems biology; Neuroscience.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Modulation of cortical slow oscillations and complexity across anesthesia levels.Neuroimage. 2021 Jan 1;224:117415. doi: 10.1016/j.neuroimage.2020.117415. Epub 2020 Oct 1. Neuroimage. 2021. PMID: 33011419
-
Attractor competition enriches cortical dynamics during awakening from anesthesia.Cell Rep. 2021 Jun 22;35(12):109270. doi: 10.1016/j.celrep.2021.109270. Cell Rep. 2021. PMID: 34161772
-
Effects of anesthesia on the response to sleep deprivation.Sleep. 2010 Dec;33(12):1659-67. doi: 10.1093/sleep/33.12.1659. Sleep. 2010. PMID: 21120128 Free PMC article.
-
Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior.Prog Brain Res. 2011;193:63-82. doi: 10.1016/B978-0-444-53839-0.00005-3. Prog Brain Res. 2011. PMID: 21854956 Review.
-
Development of coherent neuronal activity patterns in mammalian cortical networks: common principles and local hetereogeneity.Mech Dev. 2013 Jun-Aug;130(6-8):412-23. doi: 10.1016/j.mod.2012.09.006. Epub 2012 Sep 29. Mech Dev. 2013. PMID: 23032193 Review.
Cited by
-
A modular and adaptable analysis pipeline to compare slow cerebral rhythms across heterogeneous datasets.Cell Rep Methods. 2024 Jan 22;4(1):100681. doi: 10.1016/j.crmeth.2023.100681. Epub 2024 Jan 5. Cell Rep Methods. 2024. PMID: 38183979 Free PMC article.
-
In vivo imaging reveals a synchronized correlation among neurotransmitter dynamics during propofol and sevoflurane anesthesia.Zool Res. 2024 May 18;45(3):679-690. doi: 10.24272/j.issn.2095-8137.2023.302. Zool Res. 2024. PMID: 38766749 Free PMC article.
-
Topography of putative bidirectional interaction between hippocampal sharp wave ripples and neocortical slow oscillations.bioRxiv [Preprint]. 2024 Oct 23:2024.10.23.619879. doi: 10.1101/2024.10.23.619879. bioRxiv. 2024. Update in: Neuron. 2025 Mar 05;113(5):754-768.e9. doi: 10.1016/j.neuron.2024.12.019. PMID: 39484611 Free PMC article. Updated. Preprint.
-
A dietary polyphenol metabolite alters CA1 excitability ex vivo and mildly affects cortico-hippocampal field potential generators in anesthetized animals.Cereb Cortex. 2023 Sep 26;33(19):10411-10425. doi: 10.1093/cercor/bhad292. Cereb Cortex. 2023. PMID: 37550066 Free PMC article.
-
Microstimulation reveals anesthetic state-dependent effective connectivity of neurons in cerebral cortex.Front Neurosci. 2024 Jul 5;18:1387098. doi: 10.3389/fnins.2024.1387098. eCollection 2024. Front Neurosci. 2024. PMID: 39035779 Free PMC article.
References
-
- Adamantidis A.R., Gutierrez Herrera C., Gent T.C. Oscillating circuitries in the sleeping brain. Nat. Rev. Neurosci. 2019;20:746–762. - PubMed
-
- Amit D.J., Brunel N. Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb. Cortex. 1997;7:237–252. - PubMed
LinkOut - more resources
Full Text Sources

