The ARM repeat domain of hemocyanin interacts with MKK4 to modulate antimicrobial peptides expression
- PMID: 35265821
- PMCID: PMC8898971
- DOI: 10.1016/j.isci.2022.103958
The ARM repeat domain of hemocyanin interacts with MKK4 to modulate antimicrobial peptides expression
Abstract
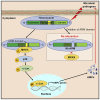
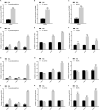
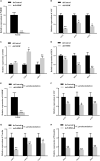
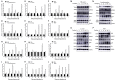
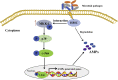
The mitogen-activated protein kinase (MAPK) intracellular signaling pathway mediates numerous biological processes, including antimicrobial immune response by inducing antimicrobial peptides (AMPs) production. Although MAPK signaling cascade proteins have been identified in penaeid shrimp, their modulation via the MKK4-p38-c-Jun cascade and effect on AMPs production is unknown. Here, we show that hemocyanin (PvHMC), antimicrobial peptides (anti-lipopolysaccharide factor, crustin, and penaeidins), and MKK4-p38-c-Jun cascade proteins are simultaneously induced by pathogens (Vibrio parahaemolyticus, Staphylococcus aureus, and white spot syndrome virus) in Penaeus vannamei. Intriguingly, knockdown of PvHMC with or without pathogen challenge attenuated the expression of MKK4-p38-c-Jun cascade proteins and their phosphorylation level, which consequently decreased AMPs expression. Further analysis revealed that PvHMC interacts via its armadillo (ARM) repeat domain with PvMKK4 to modulate the p38 MAPK signaling pathway. Thus, the ARM repeat domain enables penaeid shrimp hemocyanin to modulate AMPs expression during antimicrobial response by activating the p38 MAPK signaling pathway.
Keywords: Cell biology; Immunology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Aweya J.J., Zheng X., Zheng Z., Wang W., Fan J., Yao D., Li S., Zhang Y. The sterol regulatory element binding protein homolog of Penaeus vannamei modulates fatty acid metabolism and immune response. Biochim. Biophys. Acta Mol. Cell Biol Lipids. 2020;1865:158757. doi: 10.1016/j.bbalip.2020.158757. - DOI - PubMed
-
- Aweya J.J., Zheng Z.H., Zheng X.Y., Yao D.F., Zhang Y.L. The expanding repertoire of immune-related molecules with antimicrobial activity in penaeid shrimps: a review. Rev. Aquaculture. 2021;13:1907–1937. doi: 10.1111/raq.12551. - DOI
-
- Barreto C., Coelho J.D.R., Yuan J., Xiang J., Perazzolo L.M., Rosa R.D. Specific molecular signatures for type II crustins in penaeid shrimp uncovered by the identification of crustin-like antimicrobial peptides in litopenaeus vannamei. Mar. Drugs. 2018;16 doi: 10.3390/md16010031. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

