Understanding Contact Electrification at Water/Polymer Interface
- PMID: 35265850
- PMCID: PMC8873953
- DOI: 10.34133/2022/9861463
Understanding Contact Electrification at Water/Polymer Interface
Abstract
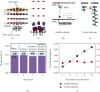
Contact electrification (CE) involves a complex interplay of physical interactions in realistic material systems. For this reason, scientific consensus on the qualitative and quantitative importance of different physical mechanisms on CE remains a formidable task. The CE mechanism at a water/polymer interface is a crucial challenge owing to the poor understanding of charge transfer at the atomic level. First-principle density functional theory (DFT), used in the present work, proposes a new paradigm to address CE. Our results indicate that CE follows the same trend as the gap between the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO) of polymers. Electron transfer occurs at the outmost atomic layer of the water/polymer interface and is closely linked to the functional groups and atom locations. When the polymer chains are parallel to the water layer, most electrons are transferred; conversely, if they are perpendicular to each other, the transfer of charges can be ignored. We demonstrate that a decrease in the interface distance between water and the polymer chains leads to CE in quantitative agreement with the electron cloud overlap model. We finally use DFT calculations to predict the properties of CE materials and their potential for triboelectric nanogenerator energy harvesting devices.
Copyright © 2022 Yang Nan et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Lowell J., Rose-Innes A. C. Contact electrification. Advances in Physics . 1980;29:947–1023. doi: 10.1080/00018738000101466. - DOI
-
- Shinbrot T., Ferdowsi B., Sundaresan S., Araujo N. A. M. Multiple timescale contact charging. Physical Review Materials . 2018;2(12, article 125003) doi: 10.1103/PhysRevMaterials.2.125003. - DOI
-
- Wang Z. L., Wang A. C. On the origin of contact-electrification. Materials Today . 2019;30:34–51. doi: 10.1016/j.mattod.2019.05.016. - DOI
-
- Shaw P. E. The electrical charges from like solids. Nature . 1926;118:659–660. doi: 10.1038/118659c0. - DOI
-
- Lacks D. J., Shinbrot T. Long-standing and unresolved issues in triboelectric charging. Nature Reviews Chemistry . 2019;3:465–476. doi: 10.1038/s41570-019-0115-1. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

