Monofractal analysis of functional magnetic resonance imaging: An introductory review
- PMID: 35266236
- PMCID: PMC9057087
- DOI: 10.1002/hbm.25801
Monofractal analysis of functional magnetic resonance imaging: An introductory review
Abstract
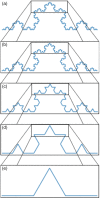
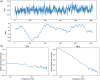
The following review will aid readers in providing an overview of scale-free dynamics and monofractal analysis, as well as its applications and potential in functional magnetic resonance imaging (fMRI) neuroscience and clinical research. Like natural phenomena such as the growth of a tree or crashing ocean waves, the brain expresses scale-invariant, or fractal, patterns in neural signals that can be measured. While neural phenomena may represent both monofractal and multifractal processes and can be quantified with many different interrelated parameters, this review will focus on monofractal analysis using the Hurst exponent (H). Monofractal analysis of fMRI data is an advanced analysis technique that measures the complexity of brain signaling by quantifying its degree of scale-invariance. As such, the H value of the blood oxygenation level-dependent (BOLD) signal specifies how the degree of correlation in the signal may mediate brain functions. This review presents a brief overview of the theory of fMRI monofractal analysis followed by notable findings in the field. Through highlighting the advantages and challenges of the technique, the article provides insight into how to best conduct fMRI fractal analysis and properly interpret the findings with physiological relevance. Furthermore, we identify the future directions necessary for its progression towards impactful functional neuroscience discoveries and widespread clinical use. Ultimately, this presenting review aims to build a foundation of knowledge among readers to facilitate greater understanding, discussion, and use of this unique yet powerful imaging analysis technique.
Keywords: Hurst exponent; complexity; fractal analysis; functional magnetic resonance imaging; neuroimaging; scale-free dynamics.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures



References
-
- Akhrif, A. , Romanos, M. , Domschke, K. , Schmitt‐Boehrer, A. , & Neufang, S. (2018). Fractal analysis of BOLD time series in a network associated with waiting impulsivity. Frontiers in Physiology, 9. 10.3389/fphys.2018.01378 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

