Analysis of relapse by inflammatory Rasch-built overall disability scale status in the PATH study of subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy
- PMID: 35266243
- PMCID: PMC9310622
- DOI: 10.1111/jns.12487
Analysis of relapse by inflammatory Rasch-built overall disability scale status in the PATH study of subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy
Abstract
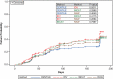
Clinical trials in chronic inflammatory demyelinating polyneuropathy (CIDP) often assess efficacy using the ordinal Inflammatory Neuropathy Cause and Treatment (INCAT) disability score. Here, data from the PATH study was reanalyzed using change in Inflammatory Rasch-built Overall Disability Scale (I-RODS) to define CIDP relapse instead of INCAT. The PATH study comprised an intravenous immunoglobulin (IVIG) dependency period and an IVIG (IgPro10 [Privigen]) restabilization period; subjects were then randomized to weekly maintenance subcutaneous immunoglobulin (SCIG; IgPro20 [Hizentra]) 0.2 g/kg or 0.4 g/kg or placebo for 24 weeks. CIDP relapse was defined as ≥1-point deterioration in adjusted INCAT, with a primary endpoint of relapse or withdrawal rates. This retrospective exploratory analysis redefined relapse using I-RODS via three different cut-off methods: an individual variability method, fixed cut-off of ≥8-point deterioration on I-RODS centile score or ≥4-point deterioration on I-RODS raw score. Relapse or withdrawal rates were 47% for placebo, 34% for 0.2 g/kg IgPro20 and 19% for 0.4 g/kg IgPro20 using the raw score; 40%, 28% and 15%, respectively using the centile score, and 49%, 40% and 27%, respectively using the individual variability method. IgPro20 was shown to be efficacious as a maintenance therapy for CIDP when relapse was defined using I-RODS. A stable response pattern was shown for I-RODS across various applied cut-offs, which could be applied in future clinical trials.
Keywords: CIDP; I-RODS; IgPro20; SCIG; chronic inflammatory demyelinating polyneuropathy; subcutaneous immunoglobulin.
© 2022 The Authors. Journal of the Peripheral Nervous System published by Wiley Periodicals LLC on behalf of Peripheral Nerve Society.
Conflict of interest statement
I. S. J. Merkies reports grants from Talecris Talents Program/Perinoms study, grants from GBS|CIDP Foundation International, grants from Prinses Beatrix Fonds, grants from European Union seventh Framework Program, other grants from Steering committee members for various studies, outside the submitted work; He serves on the editorial board of the Journal of Peripheral Nervous System, is a member of the Inflammatory Neuropathy Consortium (INC) and member of the Peripheral Nerve Society.
I. N. van Schaik chairs a steering committee for CSL Behring and received departmental honoraria for serving on scientific advisory boards for CSL Behring. He received departmental research support from The Netherlands Organization for Scientific Research and from the Dutch Prinses Beatrix Fonds. All lecturing and consulting fees for INS were donated to the Stichting Klinische Neurologie, a local foundation that supports research in the field of neurological disorders. He is a member of the Scientific Board of the Kreuth III meeting on the optimal use of plasma‐derived medicinal products, especially coagulation factors and normal immunoglobulins organized under the auspices of the European Directorate for the Quality of Medicines & HealthCare (EDQM).
V. Bril is a consultant to CSL Behring, Grifols, Pfizer, UCB, Bionevia, and ArgenX, and has received research support from Baxalta (Shire), CSL Behring, Grifols, Bionevia UCB, and ArgenX.
H‐P. Hartung has acted on steering and data monitoring committees for Bayer Healthcare, Biogen, Celgene BMS, CSL Behring, GeNeuro, Merck, Novartis, Octapharma, Receptos, Roche, Sanofi Genzyme, TG Therapeutics and VielaBio and advisory boards for Alexion and Lundbeck.
R. A. Lewis has received consultation fees and/or served on scientific advisory boards for CSL Behring, Axelacare Health Solutions, Pharnext, Biotest, Kedrion, NuFactor Inc., Optioncare and Grifols.
G. Sobue has received funds from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited, and CSL Behring.
J‐P Lawo and O. Mielke are employees of CSL Behring.
D. R. Cornblath has acted as a consultant for Acetylon Pharmaceuticals Inc., Alnylam Pharmaceuticals, Annexon Biosciences, Akros Pharma, argenx BVBA, Biotest Pharmaceuticals, Inc., Boehringer Ingelheim, Cigna Health Management, Inc., CSL Behring, DP Clinical, Inc., Grifols S.A., Hansa Medical Inc., Karos Pharmaceuticals, Inc., Merrimack Pharmaceuticals, Inc., Neurocrine Biosciences, Novartis Corp., Octapharma AG, Pharnext SAS, Seattle Genetics, Inc., Sun Pharmaceuticals and Syntimmune. He has acted on a data safety monitoring board for Pfizer Inc., Ionis Pharmaceuticals, Axovant Sciences LTD., Ampio Pharmaceuticals, PledPharma, Momenta Pharma, and Sanofi. He has a technology license with Acetylon Pharmaceuticals Inc., Akros Pharma, AstraZeneca Pharmaceuticals, LP, Calithera Biosciences, Genentech Inc, Karos Pharma, Neurocrine Biosciences, Merrimack Pharmaceuticals, Inc., Seattle Genetics, Inc. and Shire Development, LLC. He serves on the board of directors for The Peripheral Nerve Society and acts on the medical advisory board for GBS|CIDP Foundation International.
Figures

References
-
- van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35‐46. - PubMed
-
- Terhoeven P, Seybold J, Utz KS, Nickel FT, Lee DH, Linker RA. Longer‐term effects of intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: who benefits? J Neurol Sci. 2020;419:117169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

