Growth of the airway smooth muscle layer from late gestation to childhood is mediated initially by hypertrophy and subsequently hyperplasia
- PMID: 35266251
- PMCID: PMC9545757
- DOI: 10.1111/resp.14240
Growth of the airway smooth muscle layer from late gestation to childhood is mediated initially by hypertrophy and subsequently hyperplasia
Abstract
Background and objective: The airway smooth muscle (ASM) layer thickens during development. Identifying the mechanism(s) for normal structural maturation of the ASM reveals pathways susceptible to disease processes. This study characterized thickening of the ASM layer from foetal life to childhood and elucidated the underlying mechanism in terms of hypertrophy, hyperplasia and extracellular matrix (ECM) deposition.
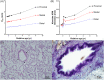
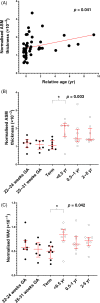
Methods: Airways from post-mortem cases were examined from seven different age groups: 22-24 weeks gestation, 25-31 weeks gestation, term (37-41 weeks gestation), <0.5 year, 0.5-1 year, 2-5 years and 6-10 years. The ASM layer area (thickness), the number and size of ASM cells and the volume fraction of ECM were assessed by planimetry and stereology.
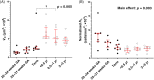
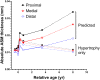
Results: From late gestation to the first year of life, normalized ASM thickness more than doubled as a result of ASM hypertrophy. Thereafter, until childhood, the ASM layer grew in proportion to airway size, which was mediated by ASM hyperplasia. Hypertrophy and hyperplasia of ASM were accompanied by a proportional change in ECM such that the broad composition of the ASM layer was constant across age groups.
Conclusion: These data suggest that the mechanisms of ASM growth from late gestation to childhood are temporally decoupled, with early hypertrophy and subsequent proliferation. We speculate that the developing airway is highly susceptible to ASM thickening in the first year of life and that the timing of an adverse event will determine structural phenotype.
Keywords: airway smooth muscle; asthma; extracellular matrix; hyperplasia; hypertrophy; ontogeny; remodelling.
© 2022 The Authors. Respirology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Respirology.
Conflict of interest statement
None declared.
Figures
Comment in
-
Development of the healthy airway smooth muscle bundles: The basis to understand airway wall remodelling.Respirology. 2022 Jul;27(7):482-483. doi: 10.1111/resp.14253. Epub 2022 Mar 30. Respirology. 2022. PMID: 35352439 No abstract available.
Similar articles
-
Airway smooth muscle hypertrophy and hyperplasia in asthma.Am J Respir Crit Care Med. 2012 May 15;185(10):1058-64. doi: 10.1164/rccm.201110-1849OC. Epub 2012 Mar 8. Am J Respir Crit Care Med. 2012. PMID: 22403800
-
Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3(beta) phosphorylation in a mouse model of asthma.Am J Physiol Lung Cell Mol Physiol. 2009 Feb;296(2):L176-84. doi: 10.1152/ajplung.90376.2008. Epub 2008 Nov 14. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19011050 Free PMC article.
-
Estimating airway smooth muscle cell volume and number in airway sections. Sources of variability.Am J Respir Cell Mol Biol. 2014 Feb;50(2):246-52. doi: 10.1165/rcmb.2013-0029OC. Am J Respir Cell Mol Biol. 2014. PMID: 24007332
-
Role of extracellular matrix and its regulators in human airway smooth muscle biology.Cell Biochem Biophys. 2006;44(1):139-46. doi: 10.1385/CBB:44:1:139. Cell Biochem Biophys. 2006. PMID: 16456242 Review.
-
Role of human airway smooth muscle in altered extracellular matrix production in asthma.Clin Exp Pharmacol Physiol. 2001 Mar;28(3):233-6. doi: 10.1046/j.1440-1681.2001.03426.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11236132 Review.
Cited by
-
Construction and validation of a nomogram model to predict bronchiolitis Mycoplasma pneumoniae pneumonia in children.Sci Rep. 2024 Dec 28;14(1):30758. doi: 10.1038/s41598-024-80906-0. Sci Rep. 2024. PMID: 39730508 Free PMC article.
References
-
- Noble PB, Jones RL, Cairncross A, Elliot JG, Mitchell HW, James AL, et al. Airway narrowing and bronchodilation to deep inspiration in bronchial segments from subjects with and without reported asthma. J Appl Physiol (1985). 2013;114:1460–71. - PubMed
-
- James AL, Bai TR, Mauad T, Abramson MJ, Dolhnikoff M, McKay KO, et al. Airway smooth muscle thickness in asthma is related to severity but not duration of asthma. Eur Respir J. 2009;34:1040–5. - PubMed
-
- Regamey N, Ochs M, Hilliard TN, Mühlfeld C, Cornish N, Fleming L, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non‐cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177:837–43. - PubMed
-
- O'Reilly R, Ullmann N, Irving S, Bossley CJ, Sonnappa S, Zhu J, et al. Increased airway smooth muscle in preschool wheezers who have asthma at school age. J Allergy Clin Immunol. 2013;131:1024–32. - PubMed
-
- Owens L, Laing IA, Zhang G, Le Souëf PN. Infant lung function predicts asthma persistence and remission in young adults. Respirology. 2017;22:289–94. - PubMed

