Synergetic integrations of bone marrow stem cells and transforming growth factor-β1 loaded chitosan nanoparticles blended silk fibroin injectable hydrogel to enhance repair and regeneration potential in articular cartilage tissue
- PMID: 35266304
- PMCID: PMC9284642
- DOI: 10.1111/iwj.13699
Synergetic integrations of bone marrow stem cells and transforming growth factor-β1 loaded chitosan nanoparticles blended silk fibroin injectable hydrogel to enhance repair and regeneration potential in articular cartilage tissue
Erratum in
-
Corrigendum.Int Wound J. 2023 Dec;20(10):4429. doi: 10.1111/iwj.14364. Epub 2023 Sep 5. Int Wound J. 2023. PMID: 37670415 Free PMC article. No abstract available.
Abstract
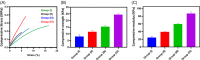
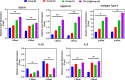
The cartilage repair and regeneration show inadequate self-healing capability and have some complications, which are inordinate challenges in clinical therapy. Biopolymeric injectable hydrogels, a prominent type of cell-carrier as well tissue engineering scaffolding materials, establish promising therapeutic potential of stem cell-based cartilage-regeneration treatment. In addition, injectable scaffolding biomaterial should have rapid gelation properties with adequate rheological and mechanical properties. In the present investigation, we developed and fabricated the macromolecular silk fibroin blended with polylysine modified chitosan polymer (SF/PCS) using thermal-sensitive glycerophosphate (GP), which contains effective gelation ability, morphology, porosity and also has enhanced mechanical properties to induce physical applicability, cell proliferation and nutrient exchange in the cell-based treatment. The developed and optimised injectable hydrogel group has good biocompatibility with human fibroblast (L929) cells and bone marrow-derived mesenchymal stem cells (BMSCs). Additionally, it was found that SF/PCS hydrogel group could sustainably release TGF-β1 and efficiently regulate cartilage-specific and inflammatory-related gene expressions. Finally, the cartilage-regeneration potential of the hydrogel groups embedded with and without BMSCs were evaluated in SD rat models under histopathological analysis, which showed promising cartilage repair. Overall, we conclude that the TGF-β1-SF/PCS injectable hydrogel demonstrates enhanced in vitro and in vivo tissue regeneration properties, which lead to efficacious therapeutic potential in cartilage regeneration.
Keywords: articular cartilage; chitosan; hydrogel; silk fibroin; stem cells.
© 2022 The Authors. International Wound Journal published by Medicalhelplines.com Inc (3M) and John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflicts of interest for the present study.
Figures









Similar articles
-
Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2.Arthritis Res Ther. 2021 Feb 2;23(1):50. doi: 10.1186/s13075-020-02382-x. Arthritis Res Ther. 2021. PMID: 33531052 Free PMC article.
-
Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration.Tissue Eng Part A. 2021 Sep;27(17-18):1213-1224. doi: 10.1089/ten.TEA.2020.0323. Epub 2021 Mar 1. Tissue Eng Part A. 2021. PMID: 33353462
-
Integration of C-type natriuretic peptide gene-modified bone marrow mesenchymal stem cells with chitosan/silk fibroin scaffolds as a promising strategy for articular cartilage regeneration.Cell Tissue Bank. 2019 Jun;20(2):209-220. doi: 10.1007/s10561-019-09760-z. Epub 2019 Mar 11. Cell Tissue Bank. 2019. PMID: 30854603
-
The preparation of silk fibroin-based hydrogels and their applications in cartilage repair.Int J Biol Macromol. 2025 May;310(Pt 4):143610. doi: 10.1016/j.ijbiomac.2025.143610. Epub 2025 Apr 27. Int J Biol Macromol. 2025. PMID: 40300680 Review.
-
Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration.Biomed Eng Online. 2025 Feb 7;24(1):13. doi: 10.1186/s12938-025-01342-3. Biomed Eng Online. 2025. PMID: 39920742 Free PMC article. Review.
Cited by
-
Chitosan alchemy: transforming tissue engineering and wound healing.RSC Adv. 2024 Jun 17;14(27):19219-19256. doi: 10.1039/d4ra01594k. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38887635 Free PMC article. Review.
-
Advanced Hydrogels With Nanoparticle Inclusion for Cartilage Tissue Engineering.Front Bioeng Biotechnol. 2022 Jun 29;10:951513. doi: 10.3389/fbioe.2022.951513. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35845428 Free PMC article. Review.
-
Chitosan-Peptide Composites for Tissue Engineering Applications: Advances in Treatment Strategies.Curr Protein Pept Sci. 2025;26(3):185-200. doi: 10.2174/0113892037323136240910052119. Curr Protein Pept Sci. 2025. PMID: 39350425 Review.
-
Preliminary study on the preparation of antler powder/chitosan/β-glycerophosphate sodium/polyvinyl alcohol porous hydrogel scaffolds and their osteogenic effects.Front Bioeng Biotechnol. 2024 Jun 26;12:1421718. doi: 10.3389/fbioe.2024.1421718. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38988866 Free PMC article.
-
Advancements in tissue engineering for articular cartilage regeneration.Heliyon. 2024 Feb 1;10(3):e25400. doi: 10.1016/j.heliyon.2024.e25400. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38352769 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

