Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II
- PMID: 35266417
- PMCID: PMC9315184
- DOI: 10.1177/13524585221078825
Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II
Abstract
Background: In the phase III ASCLEPIOS I and II trials, participants with relapsing multiple sclerosis receiving ofatumumab had significantly better clinical and magnetic resonance imaging (MRI) outcomes than those receiving teriflunomide.
Objectives: To assess the efficacy and safety of ofatumumab versus teriflunomide in recently diagnosed, treatment-naive (RDTN) participants from ASCLEPIOS.
Methods: Participants were randomized to receive ofatumumab (20 mg subcutaneously every 4 weeks) or teriflunomide (14 mg orally once daily) for up to 30 months. Endpoints analysed post hoc in the protocol-defined RDTN population included annualized relapse rate (ARR), confirmed disability worsening (CDW), progression independent of relapse activity (PIRA) and adverse events.
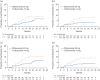
Results: Data were analysed from 615 RDTN participants (ofatumumab: n = 314; teriflunomide: n = 301). Compared with teriflunomide, ofatumumab reduced ARR by 50% (rate ratio (95% confidence interval (CI)): 0.50 (0.33, 0.74); p < 0.001), and delayed 6-month CDW by 46% (hazard ratio (HR; 95% CI): 0.54 (0.30, 0.98); p = 0.044) and 6-month PIRA by 56% (HR: 0.44 (0.20, 1.00); p = 0.049). Safety findings were manageable and consistent with those of the overall ASCLEPIOS population.
Conclusion: The favourable benefit-risk profile of ofatumumab versus teriflunomide supports its consideration as a first-line therapy in RDTN patients.ASCLEPIOS I and II are registered at ClinicalTrials.gov (NCT02792218 and NCT02792231).
Keywords: Relapsing multiple sclerosis; neurofilament light chain; no evidence of disease activity; progression independent of relapse activity; recently diagnosed; treatment-naive.
Conflict of interest statement
Figures



References
-
- Filippi M, Bar-Or A, Piehl F, et al.. Multiple sclerosis. Nat Rev Dis Primers 2018; 4(1): 43. - PubMed
-
- Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9(5): 520–532. - PubMed
-
- Scott TF. Understanding the impact of relapses in the overall course of MS; refinement of the 2 stage natural history model. J Neuroimmunol 2017; 305: 162–166. - PubMed
-
- Kappos L, Wolinsky JS, Giovannoni G, et al.. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77: 1132–1140. - PMC - PubMed

