Disentangling the Puzzling Regiochemistry of Thiol Addition to o-Quinones
- PMID: 35266705
- PMCID: PMC8981336
- DOI: 10.1021/acs.joc.1c02911
Disentangling the Puzzling Regiochemistry of Thiol Addition to o-Quinones
Abstract
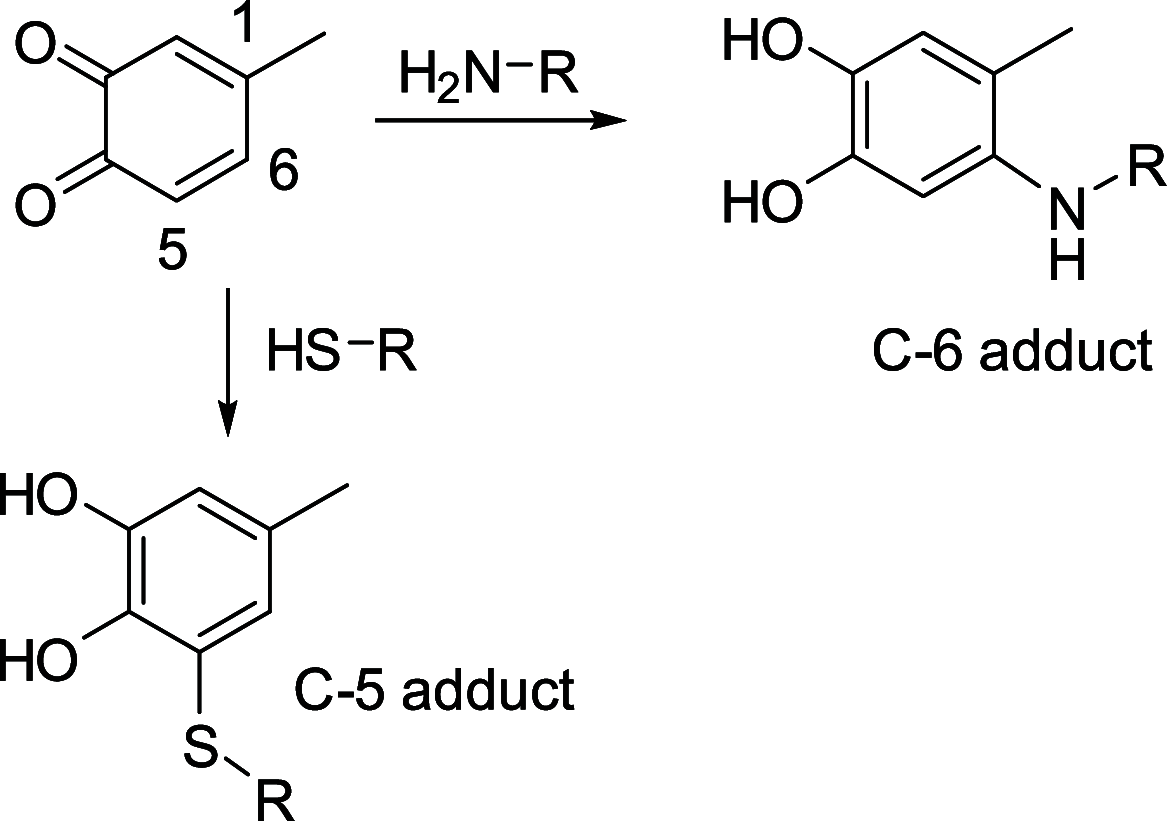
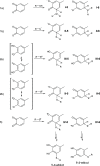
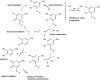
The addition of thiol compounds to o-quinones, as exemplified by the biologically relevant conjugation of cysteine to dopaquinone, displays an anomalous 1,6-type regiochemistry compared to the usual 1,4-nucleophilic addition, for example, by amines, which has so far eluded intensive investigations. By means of an integrated experimental and computational approach, herein, we provide evidence that the addition of glutathione, cysteine, or benzenethiol to 4-methyl-o-benzoquinone, modeling dopaquinone, proceeds by a free radical chain mechanism triggered by the addition of thiyl radicals to the o-quinone. In support of this conclusion, DFT calculations consistently predicted the correct regiochemistry only for the proposed thiyl radical-quinone addition pathway. These results would prompt a revision of the commonly accepted mechanisms for thiol-o-quinone conjugation and stimulate further work aimed at assessing the impact of the free radical processes in biologically relevant thiol-quinone interactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


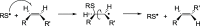


References
-
- Ito S.; Wakamatsu K.; d’Ischia M.; Napolitano A.; Pezzella A.. Structure of Melanins. In Melanins and Melanosomes; Borovansky J.; Riley P. A., Eds.; Wiley-VCH: Weinheim, 2011; pp 167–185.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

