An Optimized Dihydrodibenzothiazepine Lead Compound (SBI-0797750) as a Potent and Selective Inhibitor of Plasmodium falciparum and P. vivax Glucose 6-Phosphate Dehydrogenase 6-Phosphogluconolactonase
- PMID: 35266827
- PMCID: PMC9017341
- DOI: 10.1128/aac.02109-21
An Optimized Dihydrodibenzothiazepine Lead Compound (SBI-0797750) as a Potent and Selective Inhibitor of Plasmodium falciparum and P. vivax Glucose 6-Phosphate Dehydrogenase 6-Phosphogluconolactonase
Abstract
In Plasmodium, the first two and rate-limiting enzymes of the pentose phosphate pathway, glucose 6-phosphate dehydrogenase (G6PD) and the 6-phosphogluconolactonase, are bifunctionally fused to a unique enzyme named GluPho, differing structurally and mechanistically from the respective human orthologs. Consistent with the enzyme's essentiality for malaria parasite proliferation and propagation, human G6PD deficiency has immense impact on protection against severe malaria, making PfGluPho an attractive antimalarial drug target. Herein we report on the optimized lead compound N-(((2R,4S)-1-cyclobutyl-4-hydroxypyrrolidin-2-yl)methyl)-6-fluoro-4-methyl-11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-carboxamide (SBI-0797750), a potent and fully selective PfGluPho inhibitor with robust nanomolar activity against recombinant PfGluPho, PvG6PD, and P. falciparum blood-stage parasites. Mode-of-action studies have confirmed that SBI-0797750 disturbs the cytosolic glutathione-dependent redox potential, as well as the cytosolic and mitochondrial H2O2 homeostasis of P. falciparum blood stages, at low nanomolar concentrations. Moreover, SBI-0797750 does not harm red blood cell (RBC) integrity and phagocytosis and thus does not promote anemia. SBI-0797750 is therefore a very promising antimalarial lead compound.
Keywords: G6PDH; Plasmodium; Plasmodium falciparum; Plasmodium vivax; inhibitors; malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


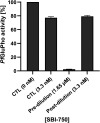


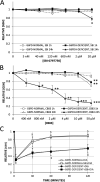


References
-
- WHO. 2020. World Malaria Report 2020. https://www.who.int/publications/i/item/9789240015791.
-
- WHO. 2017. World malaria report 2017. http://apps.who.int/iris/bitstream/handle/10665/259492/9789241565523-eng....
-
- Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio J-J, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K. 2015. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520:683–687. 10.1038/nature14412. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

