CFTR Suppresses Neointimal Formation Through Attenuating Proliferation and Migration of Aortic Smooth Muscle Cells
- PMID: 35266910
- PMCID: PMC9162269
- DOI: 10.1097/FJC.0000000000001257
CFTR Suppresses Neointimal Formation Through Attenuating Proliferation and Migration of Aortic Smooth Muscle Cells
Abstract
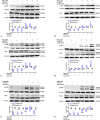
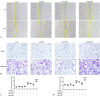
Cystic fibrosis transmembrane conductance regulator (CFTR) plays important roles in arterial functions and the fate of cells. To further understand its function in vascular remodeling, we examined whether CFTR directly regulates platelet-derived growth factor-BB (PDGF-BB)-stimulated vascular smooth muscle cells (VSMCs) proliferation and migration, as well as the balloon injury-induced neointimal formation. The CFTR adenoviral gene delivery was used to evaluate the effects of CFTR on neointimal formation in a rat model of carotid artery balloon injury. The roles of CFTR in PDGF-BB-stimulated VSMC proliferation and migration were detected by mitochondrial tetrazolium assay, wound healing assay, transwell chamber method, western blot, and qPCR. We found that CFTR expression was declined in injured rat carotid arteries, while adenoviral overexpression of CFTR in vivo attenuated neointimal formation in carotid arteries. CFTR overexpression inhibited PDGF-BB-induced VSMC proliferation and migration, whereas CFTR silencing caused the opposite results. Mechanistically, CFTR suppressed the phosphorylation of PDGF receptor β, serum and glucocorticoid-inducible kinase 1, JNK, p38 and ERK induced by PDGF-BB, and the increased mRNA expression of matrix metalloproteinase-9 and MMP2 induced by PDGF-BB. In conclusion, our results indicated that CFTR may attenuate neointimal formation by suppressing PDGF-BB-induced activation of serum and glucocorticoid-inducible kinase 1 and the JNK/p38/ERK signaling pathway.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors report no conflicts of interest.
Figures




Similar articles
-
Inhibition of p90RSK Ameliorates PDGF-BB-Mediated Phenotypic Change of Vascular Smooth Muscle Cell and Subsequent Hyperplasia of Neointima.Int J Mol Sci. 2023 Apr 30;24(9):8094. doi: 10.3390/ijms24098094. Int J Mol Sci. 2023. PMID: 37175802 Free PMC article.
-
Intermedin reduces neointima formation by regulating vascular smooth muscle cell phenotype via cAMP/PKA pathway.Atherosclerosis. 2017 Nov;266:212-222. doi: 10.1016/j.atherosclerosis.2017.10.011. Epub 2017 Oct 9. Atherosclerosis. 2017. PMID: 29053988
-
Thymoquinone suppresses platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferation, migration and neointimal formation.J Cell Mol Med. 2019 Dec;23(12):8482-8492. doi: 10.1111/jcmm.14738. Epub 2019 Oct 22. J Cell Mol Med. 2019. PMID: 31638340 Free PMC article.
-
Osteocalcin deficiency attenuates neointima formation after vascular injury in rat.BMC Cardiovasc Disord. 2025 Jul 21;25(1):534. doi: 10.1186/s12872-025-05000-3. BMC Cardiovasc Disord. 2025. PMID: 40691534 Free PMC article.
-
Galangin inhibits neointima formation induced by vascular injury via regulating the PI3K/AKT/mTOR pathway.Food Funct. 2022 Nov 28;13(23):12077-12092. doi: 10.1039/d2fo02441a. Food Funct. 2022. PMID: 36367287
Cited by
-
Evening Primrose Extracts Inhibit PDGF-BB-Induced Vascular Smooth Muscle Cell Proliferation and Migration by Regulating Cell-Cycle-Related Proteins.Curr Issues Mol Biol. 2022 Apr 27;44(5):1928-1940. doi: 10.3390/cimb44050131. Curr Issues Mol Biol. 2022. PMID: 35678660 Free PMC article.
References
-
- Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. - PubMed
-
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. - PubMed
-
- Li L, Zhang HN, Chen HZ, et al. . SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res. 2011;108:1180–1189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

