A constitutively expressed fluorescent ubiquitination-based cell-cycle indicator (FUCCI) in axolotls for studying tissue regeneration
- PMID: 35266986
- PMCID: PMC8977096
- DOI: 10.1242/dev.199637
A constitutively expressed fluorescent ubiquitination-based cell-cycle indicator (FUCCI) in axolotls for studying tissue regeneration
Abstract
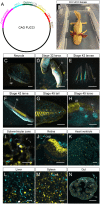
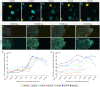
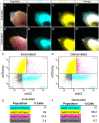
Regulation of cell cycle progression is essential for cell proliferation during regeneration following injury. After appendage amputation, the axolotl (Ambystoma mexicanum) regenerates missing structures through an accumulation of proliferating cells known as the blastema. To study cell division during blastema growth, we generated a transgenic line of axolotls that ubiquitously expresses a bicistronic version of the fluorescent ubiquitination-based cell-cycle indicator (FUCCI). We demonstrate near-ubiquitous FUCCI expression in developing and adult tissues, and validate these expression patterns with DNA synthesis and mitosis phase markers. We demonstrate the utility of FUCCI for live and whole-mount imaging, showing the predominantly local contribution of cells during limb and tail regeneration. We also show that spinal cord amputation results in increased proliferation at least 5 mm from the site of injury. Finally, we use multimodal staining to provide cell type information for cycling cells by combining fluorescence in situ hybridization, EdU click-chemistry and immunohistochemistry on a single FUCCI tissue section. This new line of animals will be useful for studying cell cycle dynamics using in situ endpoint assays and in vivo imaging in developing and regenerating animals.
Keywords: Axolotl; Cell cycle; FUCCI; Regeneration.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Alvarez, R., Jr, Wang, B. J., Quijada, P. J., Avitabile, D., Ho, T., Shaitrit, M., Chavarria, M., Firouzi, F., Ebeid, D., Monsanto, M. M.et al. (2019). Cardiomyocyte cell cycle dynamics and proliferation revealed through cardiac-specific transgenesis of fluorescent ubiquitinated cell cycle indicator (FUCCI). J. Mol. Cell. Cardiol. 127, 154-164. 10.1016/j.yjmcc.2018.12.007 - DOI - PMC - PubMed
-
- Butler, E. G. (1933). The effects of X-radiation on the regeneration of the fore limb of Amblystoma larvae. J. Exp. Zool. 65, 271-315. 10.1002/jez.1400650302 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

